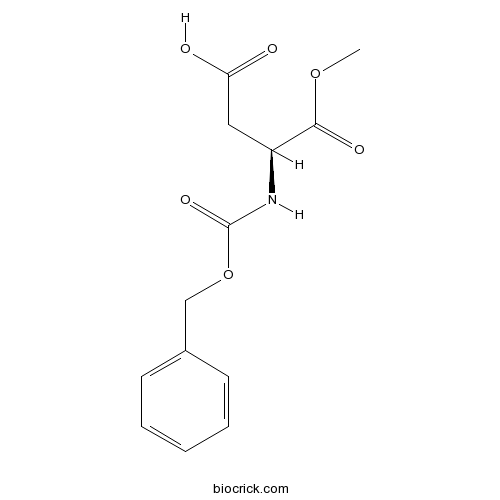
Z-Asp-OMeCAS# 4668-42-2 |
- Fulvestrant
Catalog No.:BCC1081
CAS No.:129453-61-8
- Bazedoxifene
Catalog No.:BCC1411
CAS No.:198481-32-2
- Bazedoxifene acetate
Catalog No.:BCC1412
CAS No.:198481-33-3
- (E)-2-Decenoic acid
Catalog No.:BCC1292
CAS No.:334-49-6
- Toremifene
Catalog No.:BCC2010
CAS No.:89778-26-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4668-42-2 | SDF | Download SDF |
| PubChem ID | 7021783 | Appearance | Powder |
| Formula | C13H15NO6 | M.Wt | 281.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3S)-4-methoxy-4-oxo-3-(phenylmethoxycarbonylamino)butanoic acid | ||
| SMILES | COC(=O)C(CC(=O)O)NC(=O)OCC1=CC=CC=C1 | ||
| Standard InChIKey | MFFFBNAPQRDRQW-JTQLQIEISA-N | ||
| Standard InChI | InChI=1S/C13H15NO6/c1-19-12(17)10(7-11(15)16)14-13(18)20-8-9-5-3-2-4-6-9/h2-6,10H,7-8H2,1H3,(H,14,18)(H,15,16)/t10-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Z-Asp-OMe Dilution Calculator

Z-Asp-OMe Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5549 mL | 17.7746 mL | 35.5492 mL | 71.0985 mL | 88.8731 mL |
| 5 mM | 0.711 mL | 3.5549 mL | 7.1098 mL | 14.2197 mL | 17.7746 mL |
| 10 mM | 0.3555 mL | 1.7775 mL | 3.5549 mL | 7.1098 mL | 8.8873 mL |
| 50 mM | 0.0711 mL | 0.3555 mL | 0.711 mL | 1.422 mL | 1.7775 mL |
| 100 mM | 0.0355 mL | 0.1777 mL | 0.3555 mL | 0.711 mL | 0.8887 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Z-Asp-OMe
- 3-O-(2'E ,4'E-decadienoyl)-20-O-acetylingenol
Catalog No.:BCN1437
CAS No.:466663-12-7
- 3-O-(2'E,4'E-Decadienoyl)ingenol
Catalog No.:BCN3768
CAS No.:466663-11-6
- Arteminin
Catalog No.:BCN3642
CAS No.:466639-11-2
- Cryptomeridiol
Catalog No.:BCN5516
CAS No.:4666-84-6
- Rauvomitin
Catalog No.:BCN3421
CAS No.:466-57-9
- Bullatine B
Catalog No.:BCN2375
CAS No.:466-26-2
- Benzoylaconine
Catalog No.:BCN5400
CAS No.:466-24-0
- Uzarigenin
Catalog No.:BCN5515
CAS No.:466-09-1
- Proscillaridin A
Catalog No.:BCC8239
CAS No.:466-06-8
- Hedragonic acid
Catalog No.:BCN6911
CAS No.:466-02-4
- Hederagonic acid
Catalog No.:BCN5514
CAS No.:466-01-3
- Cycloartanol
Catalog No.:BCN4860
CAS No.:4657-58-3
- N-Methylcorydiniumiodide
Catalog No.:BCN7873
CAS No.:4668-64-6
- Hecogenin
Catalog No.:BCN5408
CAS No.:467-55-0
- Coronaridine
Catalog No.:BCN3762
CAS No.:467-77-6
- Rehmannic acid
Catalog No.:BCN4632
CAS No.:467-81-2
- Theaflavin
Catalog No.:BCN5419
CAS No.:4670-05-7
- Alvespimycin
Catalog No.:BCC1346
CAS No.:467214-20-6
- 17-DMAG (Alvespimycin) HCl
Catalog No.:BCC1175
CAS No.:467214-21-7
- Diphenyleneiodonium chloride
Catalog No.:BCC6670
CAS No.:4673-26-1
- Nootkatone
Catalog No.:BCN5517
CAS No.:4674-50-4
- Lu AE58054 Hydrochloride
Catalog No.:BCC1708
CAS No.:467458-02-2
- Lu AE58054
Catalog No.:BCC1707
CAS No.:467459-31-0
- Colupulone
Catalog No.:BCN8097
CAS No.:468-27-9
Synthesis of a precursor tripeptide Z-Asp-Val-Tyr-OH of thymopentin by chemo-enzymatic method.[Pubmed:23030464]
Prep Biochem Biotechnol. 2012;42(6):520-34.
The precursor tripeptide of thymopentin was synthesized by a combination of chemical and enzymatic methods. First, Val-Tyr-OH dipeptide was synthesized by a novel chemical method in two steps involving preparation of NCA-Val. Second, the linkage of the third amino acid Z-Asp-OMe to Val-Tyr-OH was completed by an enzymatic method under kinetic control. An industrial alkaline protease alcalase was used in water-organic cosolvent systems. The synthesis reaction conditions were optimized by examining the effects of several factors including organic solvents, water content, temperature, pH, and reaction time on the yield of Z-Asp-Val-Tyr-OH. The optimum condition is of pH 10.0, 35 degrees C, acetonitrile/Na(2)CO(3)-NaHCO(3) buffer system (85:15, v/v), and reaction time of 2.5 hr, which achieves tripeptide yield of more than 70%.
Protease-catalyzed synthesis of a precursor dipeptide, Z-Asp-Val-NH2 of thymopentin, in organic solvents.[Pubmed:18800297]
Prep Biochem Biotechnol. 2008;38(4):334-47.
The protease-catalyzed, kinetically controlled synthesis of a precursor dipeptide, Z-Asp-Val-NH(2) of thymopentin (TP-5), in organic solvents was studied. Z-Asp-OMe and Val-NH(2) were used as the acyl donor and the nucleophile, respectively. An industrial alkaline protease alcalase was used to catalyze the synthesis of the target dipeptide in water-organic cosolvent systems. The conditions of the synthesis reaction were optimized by examining the effects of several factors, including organic solvents, water content, temperature, pH, and reaction time on the yield of Z-Asp-Val-NH(2). The optimum conditions using alcalase as the catalyst are pH 10.0, 35 degrees C, in acetonitrile/Na(2)CO(3)-NaHCO(3) buffer system (9:1, V/V), reaction time 5 h, with a yield of 63%. The dipeptide product was confirmed by LC- MS.
Alcalase-catalyzed, kinetically controlled synthesis of a precursor dipeptide of RGDS in organic solvents.[Pubmed:16428141]
Prep Biochem Biotechnol. 2006;36(1):93-105.
The protease-catalyzed, kinetically controlled synthesis of a precursor dipeptide of RGDS, Z-Asp-Ser-NH2 in organic solvents was studied. Alcalase, an industrial alkaline protease, was used to catalyze the synthesis of the target dipeptide in water-organic cosolvents systems with Z-Asp-OMe as the acyl donor and Ser-NH2 as the nucleophile. Acetonitrile was selected as the organic solvent from acetonitrile, ethanol, methanol, DMF, DMSO, ethyl acetate, 2-methyl-2-propanol, and chloroform tested under the experimental conditions. The conditions of the synthesis reaction were optimized by examining the effects of several factors, including water content, temperature, pH, and reaction time on the Z-Asp-Ser-NH2 yields. The optimum conditions are pH 10.0, 35 degrees C, in acetonitrile/Na2CO3-NaHCO3 buffer system (85:15, v/v), 6 h, with a dipeptide yield of 75.5%.


