BenzoylaconineCAS# 466-24-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 466-24-0 | SDF | Download SDF |
| PubChem ID | 20055771 | Appearance | Powder |
| Formula | C32H45NO10 | M.Wt | 603.7 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Isaconitine; Pikraconitin | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
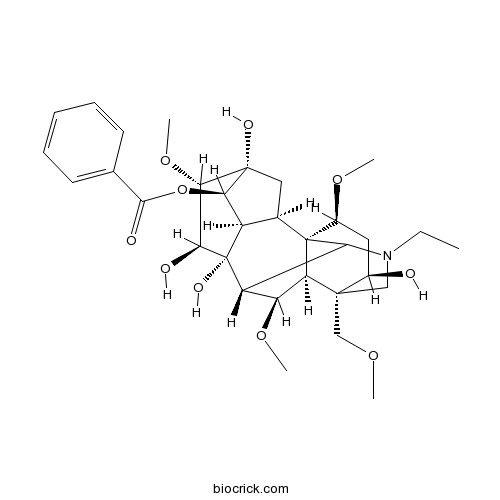
| Chemical Name | [(1S,2R,3R,4R,5R,6S,7S,8R,9R,13R,14R,16S,17S,18R)-11-ethyl-5,7,8,14-tetrahydroxy-6,16,18-trimethoxy-13-(methoxymethyl)-11-azahexacyclo[7.7.2.12,5.01,10.03,8.013,17]nonadecan-4-yl] benzoate | ||
| SMILES | CCN1CC2(C(CC(C34C2C(C(C31)C5(C6C4CC(C6OC(=O)C7=CC=CC=C7)(C(C5O)OC)O)O)OC)OC)O)COC | ||
| Standard InChIKey | DHJXZSFKLJCHLH-KYSNEVMMSA-N | ||
| Standard InChI | InChI=1S/C32H45NO10/c1-6-33-14-29(15-39-2)18(34)12-19(40-3)31-17-13-30(37)26(43-28(36)16-10-8-7-9-11-16)20(17)32(38,25(35)27(30)42-5)21(24(31)33)22(41-4)23(29)31/h7-11,17-27,34-35,37-38H,6,12-15H2,1-5H3/t17-,18-,19+,20-,21+,22+,23-,24?,25+,26-,27+,29+,30-,31+,32-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Benzoylaconine and aconitine can induce reproductive toxicity in BeWo Cell, the amino acid metabolism was the main metabolic pathway and responsible for the placental and fetal toxicity of them. |
| In vitro | Multidrug resistance-associated protein 2 is involved in the efflux of Aconitum alkaloids determined by MRP2-MDCKII cells.[Pubmed: 25744397]Life Sci. 2015 Apr 15;127:66-72.Aconitum alkaloids mainly contain highly toxic aconitine (AC), mesaconitine (MA), and hypaconitine (HA) and less toxic Benzoylaconine (BAC), benzoylmesaconine (BMA), benzoylhypaconine (BHA), aconine, mesaconine, and hypaconine. The efflux transporters including P-glycoprotein (P-gp), breast cancer resistance protein (BCRP) and multidrug resistance-associated protein 2 (MRP2) can efflux toxicants to prevent poisoning. Our previous publication has proved that P-gp and BCRP contributed to the efflux of AC, MA and HA, which is demonstrated in the human colonic adenocarcinoma cell lines (Caco-2 cells), Mardin-Darby canine kidney cell lines transfected with MDR1 or BCRP (MDR1-MDCKII and BCRP-MDCKII cells). However, the role of MRP2 remains uncertain.
|
| Cell Research | Metabolomics Study of Aconitine and Benzoylaconine Induced Reproductive Toxicity in BeWo Cell. [Reference: WebLink]Chinese J. Anal. Chem., 2015, 43(12):1808-13.An intracellular metabolomics method based on gas chromatography coupled with mass spectrometry (GC-MS) was established to explore the toxicity mechanism of aconitine and Benzoylaconine. BeWo, a continuous cell lines originated from human placenta, was selected to establish in vitro placenta model.
|

Benzoylaconine Dilution Calculator

Benzoylaconine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6565 mL | 8.2823 mL | 16.5645 mL | 33.129 mL | 41.4113 mL |
| 5 mM | 0.3313 mL | 1.6565 mL | 3.3129 mL | 6.6258 mL | 8.2823 mL |
| 10 mM | 0.1656 mL | 0.8282 mL | 1.6565 mL | 3.3129 mL | 4.1411 mL |
| 50 mM | 0.0331 mL | 0.1656 mL | 0.3313 mL | 0.6626 mL | 0.8282 mL |
| 100 mM | 0.0166 mL | 0.0828 mL | 0.1656 mL | 0.3313 mL | 0.4141 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Benzoylaconine(Isaconitine; Pikraconitin) is an alkaloid in the Chinese traditional medicine Radix Aconiti Lateralis Preparata (Fuzi).
References:
[1]. Liu XX, et al. Determination of alkaloids in Radix Aconiti Lateralis Preparata by RP-ion-pair HPLC. Yao Xue Xue Bao. 2006 Apr;41(4):365-9.
[2]. Borcsa B, et al. Diterpene alkaloids from the roots of Aconitum moldavicum and assessment of Nav1.2 sodium channel activity of aconitum alkaloids. Planta Med. 2014 Feb;80(2-3):231-6.
- Uzarigenin
Catalog No.:BCN5515
CAS No.:466-09-1
- Proscillaridin A
Catalog No.:BCC8239
CAS No.:466-06-8
- Hedragonic acid
Catalog No.:BCN6911
CAS No.:466-02-4
- Hederagonic acid
Catalog No.:BCN5514
CAS No.:466-01-3
- Cycloartanol
Catalog No.:BCN4860
CAS No.:4657-58-3
- alpha-Spinasterol acetate
Catalog No.:BCN5510
CAS No.:4651-46-1
- Hederagenin
Catalog No.:BCN5513
CAS No.:465-99-6
- Marrubiin
Catalog No.:BCC8208
CAS No.:465-92-9
- Quinovic acid
Catalog No.:BCN5512
CAS No.:465-74-7
- Resibufogenin
Catalog No.:BCN5366
CAS No.:465-39-4
- Bufalin
Catalog No.:BCN1046
CAS No.:465-21-4
- Polyporenic acid C
Catalog No.:BCN3645
CAS No.:465-18-9
- Bullatine B
Catalog No.:BCN2375
CAS No.:466-26-2
- Rauvomitin
Catalog No.:BCN3421
CAS No.:466-57-9
- Cryptomeridiol
Catalog No.:BCN5516
CAS No.:4666-84-6
- Arteminin
Catalog No.:BCN3642
CAS No.:466639-11-2
- 3-O-(2'E,4'E-Decadienoyl)ingenol
Catalog No.:BCN3768
CAS No.:466663-11-6
- 3-O-(2'E ,4'E-decadienoyl)-20-O-acetylingenol
Catalog No.:BCN1437
CAS No.:466663-12-7
- Z-Asp-OMe
Catalog No.:BCC2792
CAS No.:4668-42-2
- N-Methylcorydiniumiodide
Catalog No.:BCN7873
CAS No.:4668-64-6
- Hecogenin
Catalog No.:BCN5408
CAS No.:467-55-0
- Coronaridine
Catalog No.:BCN3762
CAS No.:467-77-6
- Rehmannic acid
Catalog No.:BCN4632
CAS No.:467-81-2
- Theaflavin
Catalog No.:BCN5419
CAS No.:4670-05-7
Multidrug resistance-associated protein 2 is involved in the efflux of Aconitum alkaloids determined by MRP2-MDCKII cells.[Pubmed:25744397]
Life Sci. 2015 Apr 15;127:66-72.
AIMS: Aconitum alkaloids mainly contain highly toxic aconitine (AC), mesaconitine (MA), and hypaconitine (HA) and less toxic Benzoylaconine (BAC), benzoylmesaconine (BMA), benzoylhypaconine (BHA), aconine, mesaconine, and hypaconine. The efflux transporters including P-glycoprotein (P-gp), breast cancer resistance protein (BCRP) and multidrug resistance-associated protein 2 (MRP2) can efflux toxicants to prevent poisoning. Our previous publication has proved that P-gp and BCRP contributed to the efflux of AC, MA and HA, which is demonstrated in the human colonic adenocarcinoma cell lines (Caco-2 cells), Mardin-Darby canine kidney cell lines transfected with MDR1 or BCRP (MDR1-MDCKII and BCRP-MDCKII cells). However, the role of MRP2 remains uncertain. MAIN METHODS: The MRP2-MDCKII cells were used to determine the efflux ratios (Er) and intracellular amounts of Aconitum alkaloids. In addition, the importance of MRP2 was further investigated with or without the MRP2 inhibitor, LTC4. KEY FINDINGS: The Er values of AC, MA, HA, BAC, BMA and BHA in MRP2-MDCKII cells (6.4 +/- 0.3, 5.9 +/- 0.5, 2.2 +/- 0.2, 1.6 +/- 0.3, 1.7 +/- 0.2 and 1.9 +/- 0.2 respectively) were significantly higher than those in MDCKII cells, which were close to 1. In the presence of LTC4, the Er values of AC, MA, HA, BAC, BMA and BHA were reduced to approximately 1 and their intracellular amounts were also significantly increased in MRP2-MDCKII cells. SIGNIFICANCE: MRP2 was involved in the efflux of AC, MA, HA, BAC, BMA and BHA, which would be useful for the safe application of these components or their herbs.


