UBP 296CAS# 745055-86-1 |
- Laminin (925-933)
Catalog No.:BCC1015
CAS No.:110590-60-8
- Epidermal Growth Factor Receptor Peptide (985-996)
Catalog No.:BCC1014
CAS No.:96249-43-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 745055-86-1 | SDF | Download SDF |
| PubChem ID | 11674376 | Appearance | Powder |
| Formula | C15H15N3O6 | M.Wt | 333.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in 1eq. NaOH with gentle warming and to 10 mM in DMSO | ||
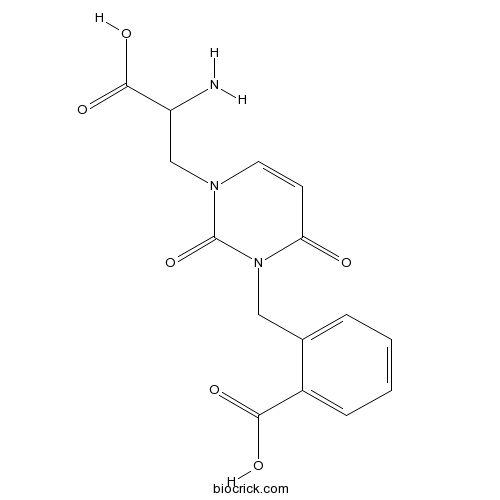
| Chemical Name | 2-[[3-(2-amino-2-carboxyethyl)-2,6-dioxopyrimidin-1-yl]methyl]benzoic acid | ||
| SMILES | C1=CC=C(C(=C1)CN2C(=O)C=CN(C2=O)CC(C(=O)O)N)C(=O)O | ||
| Standard InChIKey | UUIYULWYHDSXHL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H15N3O6/c16-11(14(22)23)8-17-6-5-12(19)18(15(17)24)7-9-3-1-2-4-10(9)13(20)21/h1-6,11H,7-8,16H2,(H,20,21)(H,22,23) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective GluR5-subunit containing kainate receptor antagonist (apparent KD = 1.09 μM). Displays ~ 90-fold selectivity over AMPA receptors and recombinant human GluR6- and KA2-containing kainate receptors. Has little or no action at NMDA or group I mGlu receptors. Selectively blocks kainate receptor-mediated LTP induction in rat hippocampal mossy fibers. Active enantiomer UBP 302 available. |

UBP 296 Dilution Calculator

UBP 296 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0003 mL | 15.0015 mL | 30.003 mL | 60.006 mL | 75.0075 mL |
| 5 mM | 0.6001 mL | 3.0003 mL | 6.0006 mL | 12.0012 mL | 15.0015 mL |
| 10 mM | 0.3 mL | 1.5002 mL | 3.0003 mL | 6.0006 mL | 7.5008 mL |
| 50 mM | 0.06 mL | 0.3 mL | 0.6001 mL | 1.2001 mL | 1.5002 mL |
| 100 mM | 0.03 mL | 0.15 mL | 0.3 mL | 0.6001 mL | 0.7501 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ivachtin
Catalog No.:BCC2357
CAS No.:745046-84-8
- MMAF
Catalog No.:BCC5144
CAS No.:745017-94-1
- Alprostadil
Catalog No.:BCC4963
CAS No.:745-65-3
- Casegravol
Catalog No.:BCN4591
CAS No.:74474-76-3
- Potassium Chloride
Catalog No.:BCC7581
CAS No.:7447-40-7
- Sauvagine
Catalog No.:BCC5792
CAS No.:74434-59-6
- Symlandine
Catalog No.:BCN1974
CAS No.:74410-74-5
- PAF (C16)
Catalog No.:BCC7522
CAS No.:74389-68-7
- Leuprolide Acetate
Catalog No.:BCC1701
CAS No.:74381-53-6
- 8-Geranyloxypsoralen
Catalog No.:BCN4296
CAS No.:7437-55-0
- 4-hydroxyephedrine hydrochloride
Catalog No.:BCC8103
CAS No.:7437-54-9
- Ent-16Α,17-Dihydroxy-19-Kauranoic Acid
Catalog No.:BCC9227
CAS No.:74365-74-5
- UBP 302
Catalog No.:BCC7256
CAS No.:745055-91-8
- Cristacarpin
Catalog No.:BCN3935
CAS No.:74515-47-2
- Isomedicarpin
Catalog No.:BCN4297
CAS No.:74560-05-7
- D-64131
Catalog No.:BCC1510
CAS No.:74588-78-6
- Rubiadin 1-methyl ether
Catalog No.:BCN4298
CAS No.:7460-43-7
- Tetrahydrocoptisine
Catalog No.:BCN2558
CAS No.:7461-02-1
- Tylosin tartrate
Catalog No.:BCC4875
CAS No.:74610-55-2
- ent-3Beta-Angeloyloxykaur-16-en-19-oic acid
Catalog No.:BCN1365
CAS No.:74635-61-3
- 11-Hydroxydrim-7-en-6-one
Catalog No.:BCN7770
CAS No.:74635-87-3
- Liquiritin Apioside
Catalog No.:BCC8334
CAS No.:74639-14-8
- 1,3,9-Trimethyluric acid
Catalog No.:BCN7393
CAS No.:7464-93-9
- Nuclear yellow
Catalog No.:BCC1810
CAS No.:74681-68-8
Ethanol inhibition of kainate receptor-mediated excitatory neurotransmission in the rat basolateral nucleus of the amygdala.[Pubmed:18617194]
Neuropharmacology. 2008 Oct;55(5):661-8.
The neurobiological mechanisms governing alcohol-induced alterations in anxiety-like behaviors are not fully understood. Given that the amygdala is a major emotional center in the brain and regulates the expression of both learned fear and anxiety, neurotransmitter systems within the basolateral amygdala represent likely mechanisms governing the anxiety-related effects of acute ethanol exposure. It is well established that, within the glutamatergic system, N-methyl-d-aspartate (NMDA)-type receptors are particularly sensitive to intoxicating concentrations of ethanol. However, recent evidence suggests that kainate-type glutamate receptors are sensitive to ethanol as well. Therefore, we examined the effect of acute ethanol on kainate receptor (KA-R)-mediated synaptic transmission in the basolateral amygdala (BLA) of Sprague-Dawley rats. Acute ethanol decreased KA-R-mediated excitatory postsynaptic currents (EPSCs) in the BLA in a concentration-dependent manner. Ethanol also inhibited currents evoked by focal application of the kainate receptor agonist (R,S)-2-amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl) propanoic acid (ATPA), and ethanol inhibition of kainate EPSCs was not associated with a change in paired-pulse ratio, suggesting a postsynaptic mechanism of ethanol action. The neurophysiological consequences of this acute sensitivity were tested by measuring ethanol's effects on KA-R-dependent modulation of synaptic plasticity. Acute ethanol, like the GluR5-specific antagonist (R,S)-3-(2-carboxybenzyl)willardiine (UBP 296), robustly diminished ATPA-induced increases in synaptic efficacy. Lastly, to better understand the relationship between KA-R activity and anxiety-like behavior, we bilaterally microinjected ATPA directly into the BLA. We observed an increase in measures of anxiety-like behavior, assessed in the light/dark box, with no change in locomotor activity. This evidence suggests that kainate receptors in the BLA are inhibited by pharmacologically relevant concentrations of ethanol and may contribute to some of the acute anxiolytic effects of this drug.
Synthesis and pharmacology of willardiine derivatives acting as antagonists of kainate receptors.[Pubmed:16302825]
J Med Chem. 2005 Dec 1;48(24):7867-81.
The natural product willardiine (8) is an AMPA receptor agonist while 5-iodowillardiine (10) is a selective kainate receptor agonist. In an attempt to produce antagonists of kainate and AMPA receptors analogues of willardiine with substituents at the N3 position of the uracil ring were synthesized. The N3-4-carboxybenzyl substituted analogue (38c) was found to be equipotent at AMPA and GLUK5-containing kainate receptors in the neonatal rat spinal cord. The N3-2-carboxybenzyl substituted analogue (38a) proved to be a potent and selective GLUK5 subunit containing kainate receptor antagonist when tested on native rat and human recombinant AMPA and kainate receptor subtypes. The GLUK5 kainate receptor antagonist activity was found to reside in the S enantiomer (44a) whereas the R enantiomer (44b) was almost inactive. 5-Iodo substitution of the uracil ring of 44a gave 45, which was found to have enhanced potency and selectivity for GLUK5.
Characterisation of UBP296: a novel, potent and selective kainate receptor antagonist.[Pubmed:15165833]
Neuropharmacology. 2004 Jul;47(1):46-64.
Willardiine derivatives with an N3-benzyl substituent bearing an acidic group have been synthesized with the aim of producing selective antagonists for GLUK5-containing kainate receptors. UBP296 was found to be a potent and selective antagonist of native GLUK5-containing kainate receptors in the spinal cord, with activity residing in the S enantiomer (UBP302). In cells expressing human kainate receptor subunits, UBP296 selectively depressed glutamate-induced calcium influx in cells containing GLUK5 in homomeric or heteromeric forms. In radioligand displacement binding studies, the willardiine analogues displaced [3H]kainate binding with IC50 values >100 microM at rat GLUK6, GLUK2 or GLUK6/GLUK2. An explanation of the GLUK5 selectivity of UBP296 was obtained using homology models of the antagonist bound forms of GLUK5 and GLUK6. In rat hippocampal slices, UBP296 reversibly blocked ATPA-induced depressions of synaptic transmission at concentrations subthreshold for affecting AMPA receptor-mediated synaptic transmission directly. UBP296 also completely blocked the induction of mossy fibre LTP, in medium containing 2 mM (but not 4 mM) Ca2+. These data provide further evidence for a role for GLUK5-containing kainate receptors in mossy fibre LTP. In conclusion, UBP296 is the most potent and selective antagonist of GLUK5-containing kainate receptors so far described.


