MMAFAnti-mitotic/anti-tubulin/antineoplastic agent CAS# 745017-94-1 |
- Mc-MMAD
Catalog No.:BCC1735
CAS No.:1401963-15-2
- Nocodazole
Catalog No.:BCC3826
CAS No.:31430-18-9
- Colchicine
Catalog No.:BCN6271
CAS No.:64-86-8
- Mc-MMAE
Catalog No.:BCC5201
CAS No.:863971-24-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 745017-94-1 | SDF | Download SDF |
| PubChem ID | 10395173 | Appearance | Powder |
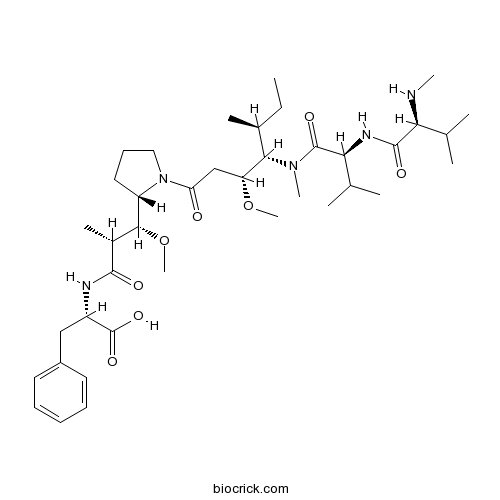
| Formula | C39H65N5O8 | M.Wt | 731.96 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Monomethylauristatin F | ||
| Solubility | >36.6mg/mL in DMSO | ||
| Chemical Name | (2S)-2-[[(2R,3R)-3-methoxy-3-[(2S)-1-[(3R,4S,5S)-3-methoxy-5-methyl-4-[methyl-[(2S)-3-methyl-2-[[(2S)-3-methyl-2-(methylamino)butanoyl]amino]butanoyl]amino]heptanoyl]pyrrolidin-2-yl]-2-methylpropanoyl]amino]-3-phenylpropanoic acid | ||
| SMILES | CCC(C)C(C(CC(=O)N1CCCC1C(C(C)C(=O)NC(CC2=CC=CC=C2)C(=O)O)OC)OC)N(C)C(=O)C(C(C)C)NC(=O)C(C(C)C)NC | ||
| Standard InChIKey | MFRNYXJJRJQHNW-DEMKXPNLSA-N | ||
| Standard InChI | InChI=1S/C39H65N5O8/c1-12-25(6)34(43(9)38(48)33(24(4)5)42-37(47)32(40-8)23(2)3)30(51-10)22-31(45)44-20-16-19-29(44)35(52-11)26(7)36(46)41-28(39(49)50)21-27-17-14-13-15-18-27/h13-15,17-18,23-26,28-30,32-35,40H,12,16,19-22H2,1-11H3,(H,41,46)(H,42,47)(H,49,50)/t25-,26+,28-,29-,30+,32-,33-,34-,35+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | MMAF is an antitubulin agent that inhibit cell division; inhibits H3397 cell growth with an IC50 of 105 nM.In Vitro:MMAF shows in vitro cytotoxicity against a panel of cell lines. The IC50 values for Karpas 299, H3396, 786-O and Caki-1 are 119, 105, 257, and 200 nM, respectively. Targeted MMAF is much more potent than the free drug, and that cAC10 conjugates of MMAF display pronounced activities. On a molar basis, the cAC10-L1-MMAF4 is an average of over 2200-fold more potent than free MMAF and is active on all the CD30-positive cell lines tested[1].In Vivo:The maximum tolerated dose in mice of MMAF (>16 mg/kg) is much higher than MMAE (1 mg/kg). cAC10-L1-MMAF4 has an MTD of 50 mg/kg in mice and 15 mg/kg in rats. The corresponding cAC10-L4-MMAF4 ADC was much less toxic, having MTDs in mice and rats of >150 mg/ kg and 90 mg/kg in rats, respectively[1]. References: | |||||

MMAF Dilution Calculator

MMAF Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3662 mL | 6.831 mL | 13.6619 mL | 27.3239 mL | 34.1549 mL |
| 5 mM | 0.2732 mL | 1.3662 mL | 2.7324 mL | 5.4648 mL | 6.831 mL |
| 10 mM | 0.1366 mL | 0.6831 mL | 1.3662 mL | 2.7324 mL | 3.4155 mL |
| 50 mM | 0.0273 mL | 0.1366 mL | 0.2732 mL | 0.5465 mL | 0.6831 mL |
| 100 mM | 0.0137 mL | 0.0683 mL | 0.1366 mL | 0.2732 mL | 0.3415 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
MMAF is a highly potent antimitotic agent [1].
MMAF is an auristatin F (AF) derivative and is used as an antimitotic drug in the form of antibody drug conjugates (ADCs). It is reported that both the N-terminal monomethylvaline and the C-terminal carboxyl group of MMAF can be attached to the linker. Dipeptide linkers used for the attachment of MMAF to mAbs result in conjugates pronounced and potent activities against an array of tumor types. In the in vitro cytotoxicity assays, the ADC generated by attaching MMAF to the 1F6 mAb shows a potent inhibition to the cell viability with IC50 values of 10ng/ml, 8ng/ml, and 123ng/ml in 786-O, Caki-1 and L428 cell lines, respectively. Moreover, it is reported that the dipeptide linker attached to MMAF with C-terminal amino acids could modulate the efficacy, potency, and tolerability. The manipulation of the C-terminal peptide sequence leads to the improved potency, specificity and therapeutic windows of the conjugates [1].
References:
[1] Doronina SO, Bovee TD, Meyer DW, Miyamoto JB, Anderson ME, Morris-Tilden CA, Senter PD. Novel peptide linkers for highly potent antibody-auristatin conjugate. Bioconjug Chem. 2008 Oct;19(10):1960-3.
- Alprostadil
Catalog No.:BCC4963
CAS No.:745-65-3
- Casegravol
Catalog No.:BCN4591
CAS No.:74474-76-3
- Potassium Chloride
Catalog No.:BCC7581
CAS No.:7447-40-7
- Sauvagine
Catalog No.:BCC5792
CAS No.:74434-59-6
- Symlandine
Catalog No.:BCN1974
CAS No.:74410-74-5
- PAF (C16)
Catalog No.:BCC7522
CAS No.:74389-68-7
- Leuprolide Acetate
Catalog No.:BCC1701
CAS No.:74381-53-6
- 8-Geranyloxypsoralen
Catalog No.:BCN4296
CAS No.:7437-55-0
- 4-hydroxyephedrine hydrochloride
Catalog No.:BCC8103
CAS No.:7437-54-9
- Ent-16Α,17-Dihydroxy-19-Kauranoic Acid
Catalog No.:BCC9227
CAS No.:74365-74-5
- Chidamide
Catalog No.:BCC6445
CAS No.:743420-02-2
- (RS)-AMPA
Catalog No.:BCC6560
CAS No.:74341-63-2
- Ivachtin
Catalog No.:BCC2357
CAS No.:745046-84-8
- UBP 296
Catalog No.:BCC7255
CAS No.:745055-86-1
- UBP 302
Catalog No.:BCC7256
CAS No.:745055-91-8
- Cristacarpin
Catalog No.:BCN3935
CAS No.:74515-47-2
- Isomedicarpin
Catalog No.:BCN4297
CAS No.:74560-05-7
- D-64131
Catalog No.:BCC1510
CAS No.:74588-78-6
- Rubiadin 1-methyl ether
Catalog No.:BCN4298
CAS No.:7460-43-7
- Tetrahydrocoptisine
Catalog No.:BCN2558
CAS No.:7461-02-1
- Tylosin tartrate
Catalog No.:BCC4875
CAS No.:74610-55-2
- ent-3Beta-Angeloyloxykaur-16-en-19-oic acid
Catalog No.:BCN1365
CAS No.:74635-61-3
- 11-Hydroxydrim-7-en-6-one
Catalog No.:BCN7770
CAS No.:74635-87-3
- Liquiritin Apioside
Catalog No.:BCC8334
CAS No.:74639-14-8
A high current pulsed power generator CQ-3-MMAF with co-axial cable transmitting energy for material dynamics experiments.[Pubmed:27370784]
Rev Sci Instrum. 2016 Jun;87(6):065110.
A high current pulsed power generator CQ-3-MMAF (Multi-Modules Assembly Facility, MMAF) was developed for material dynamics experiments under ramp wave and shock loadings at the Institute of Fluid Physics (IFP), which can deliver 3 MA peak current to a strip-line load. The rise time of the current is 470 ns (10%-90%). Different from the previous CQ-4 at IFP, the CQ-3-MMAF energy is transmitted by hundreds of co-axial high voltage cables with a low impedance of 18.6 mOmega and low loss, and then hundreds of cables are reduced and converted to tens of cables into a vacuum chamber by a cable connector, and connected with a pair of parallel metallic plates insulated by Kapton films. It is composed of 32 capacitor and switch modules in parallel. The electrical parameters in short circuit are with a capacitance of 19.2 muF, an inductance of 11.7 nH, a resistance of 4.3 mOmega, and working charging voltage of 60 kV-90 kV. It can be run safely and stable when charged from 60 kV to 90 kV. The vacuum of loading chamber can be up to 10(-2) Pa, and the current waveforms can be shaped by discharging in time sequences of four groups of capacitor and switch modules. CQ-3-MMAF is an adaptive machine with lower maintenance because of its modularization design. The COMSOL Multi-physics(R) code is used to optimize the structure of some key components and calculate their structural inductance for designs, such as gas switches and cable connectors. Some ramp wave loading experiments were conducted to check and examine the performances of CQ-3-MMAF. Two copper flyer plates were accelerated to about 3.5 km/s in one shot when the working voltage was charged to 70 kV. The velocity histories agree very well. The dynamic experiments of some polymer bonded explosives and phase transition of tin under ramp wave loadings were also conducted. The experimental data show that CQ-3-MMAF can be used to do material dynamics experiments in high rate and low cost shots. Based on this design concept, the peak current of new generators can be increased to 5-6 MA and about 100 GPa ramp stress can be produced on the metallic samples for high pressure physics, and a conceptual design of CQ-5-MMAF was given.
Whole-exome sequencing of familial cases of multiple morphological abnormalities of the sperm flagella (MMAF) reveals new DNAH1 mutations.[Pubmed:27798045]
Hum Reprod. 2016 Dec;31(12):2872-2880.
STUDY QUESTION: Can whole-exome sequencing (WES) of patients with multiple morphological abnormalities of the sperm flagella (MMAF) identify causal mutations in new genes or mutations in the previously identified dynein axonemal heavy chain 1 (DNAH1) gene? SUMMARY ANSWER: WES for six families with men affected by MMAF syndrome allowed the identification of DNAH1 mutations in four affected men distributed in two out of the six families but no new candidate genes were identified. WHAT IS KNOWN ALREADY: Mutations in DNAH1, an axonemal inner dynein arm heavy chain gene, have been shown to be responsible for male infertility due to a characteristic form of asthenozoospermia called MMAF, defined by the presence in the ejaculate of spermatozoa with a mosaic of flagellar abnormalities including absent, coiled, bent, angulated, irregular and short flagella. STUDY DESIGN, SIZE, DURATION: This was a retrospective genetics study of patients presenting a MMAF phenotype. Patients were recruited in Iran and Italy between 2008 and 2015. PARTICIPANTS/MATERIALS, SETTING, METHODS: WES was performed for a total of 10 subjects. All identified variants were confirmed by Sanger sequencing. Two additional affected family members were analyzed by direct Sanger sequencing. To establish the prevalence of the DNAH1 mutation identified in an Iranian family, we carried out targeted sequencing on 38 additional MMAF patients of the same geographical origin. RT-PCR and immunochemistry were performed on sperm samples to assess the effect of the identified mutation on RNA and protein. MAIN RESULTS AND THE ROLE OF CHANCE: WES in six families identified a causal mutations in two families. Two additional affected family members were confirmed to hold the same homozygous mutation as their sibling. In total, DNAH1 mutations were identified in 5 out of 12 analyzed subjects (41.7%). If we only include index cases, we detected two mutated subjects out of six (33%) tested MMAF individuals. Furthermore we sequenced one DNAH1 exon found to be mutated (c.8626-1G > A) in an Iranian family in an additional 38 MMAF patients from Iran. One of these patients carried the variant confirming that this variant is relatively frequent in the Iranian population. The effect of the c.8626-1G > A variant was confirmed by RT-PCR and immunochemistry as no RNA or protein could be observed in sperm from the affected men. LARGE SCALE DATA: N/A. LIMITATIONS, REASONS FOR CAUTION: WES allows the amplification of 80-90% of all coding exons. It is possible that some DNAH1 exons may not have been sequenced and that we may have missed some additional mutations. Also, WES cannot identify deep intronic mutations and it is not efficient for detection of large genomic events (deletions, insertions, inversions). We did not identify any causal mutations in DNAH1 or in other candidate genes in four out of the six tested families. This indicates that the technique and/or the analysis of our data can be improved to increase the diagnosis efficiency. WIDER IMPLICATIONS OF THE FINDINGS: Our findings confirm that DNAH1 is one of the main genes involved in MMAF syndrome. It is a large gene with 78 exons making it challenging and expensive to sequence using the traditional Sanger sequencing methods. We show that WES sequencing is good alternative to Sanger sequencing to reach a genetic diagnosis in patients with severe male infertility phenotypes. STUDY FUNDING/COMPETING INTERESTS: This work was supported by following grants: the 'MAS-Flagella' project financed by the French ANR and the DGOS for the program PRTS 2014 and the 'Whole genome sequencing of patients with Flagellar Growth Defects (FGD)' project financed by the Fondation Maladies Rares for the program Sequencage a haut debit 2012. The authors have no conflict of interest.
Therapeutic Efficacy of a Family of pHLIP-MMAF Conjugates in Cancer Cells and Mouse Models.[Pubmed:28048942]
Mol Pharm. 2017 Feb 6;14(2):415-422.
The targeting of therapeutics specifically to diseased tissue is crucial for the development of successful cancer treatments. The approach here is based on the pH(low) insertion peptide (pHLIP) for the delivery of a potent mitotic inhibitor monomethyl auristatin F (MMAF). We investigated six pHLIP variants conjugated to MMAF to compare their efficacy in vitro against cultured cancer cells. While all pHLIP-MMAF conjugates exhibit potent pH- and concentration-dependent killing, their cytotoxicity profiles are remarkably different. We also show that the lead conjugate exhibits significant therapeutic efficacy in mouse models without overt toxicities. This study confirms pHLIP-monomethyl auristatin conjugates as possible new therapeutic options for cancer treatment and supports their further development.
Targeted Drug Delivery with an Integrin-Binding Knottin-Fc-MMAF Conjugate Produced by Cell-Free Protein Synthesis.[Pubmed:27197305]
Mol Cancer Ther. 2016 Jun;15(6):1291-300.
Antibody-drug conjugates (ADC) have generated significant interest as targeted therapeutics for cancer treatment, demonstrating improved clinical efficacy and safety compared with systemic chemotherapy. To extend this concept to other tumor-targeting proteins, we conjugated the tubulin inhibitor monomethyl-auristatin-F (MMAF) to 2.5F-Fc, a fusion protein composed of a human Fc domain and a cystine knot (knottin) miniprotein engineered to bind with high affinity to tumor-associated integrin receptors. The broad expression of integrins (including alphavbeta3, alphavbeta5, and alpha5beta1) on tumor cells and their vasculature makes 2.5F-Fc an attractive tumor-targeting protein for drug delivery. We show that 2.5F-Fc can be expressed by cell-free protein synthesis, during which a non-natural amino acid was introduced into the Fc domain and subsequently used for site-specific conjugation of MMAF through a noncleavable linker. The resulting knottin-Fc-drug conjugate (KFDC), termed 2.5F-Fc-MMAF, had approximately 2 drugs attached per KFDC. 2.5F-Fc-MMAF inhibited proliferation in human glioblastoma (U87MG), ovarian (A2780), and breast (MB-468) cancer cells to a greater extent than 2.5F-Fc or MMAF alone or added in combination. As a single agent, 2.5F-Fc-MMAF was effective at inducing regression and prolonged survival in U87MG tumor xenograft models when administered at 10 mg/kg two times per week. In comparison, tumors treated with 2.5F-Fc or MMAF were nonresponsive, and treatment with a nontargeted control, CTRL-Fc-MMAF, showed a modest but not significant therapeutic effect. These studies provide proof-of-concept for further development of KFDCs as alternatives to ADCs for tumor targeting and drug delivery applications. Mol Cancer Ther; 15(6); 1291-300. (c)2016 AACR.


