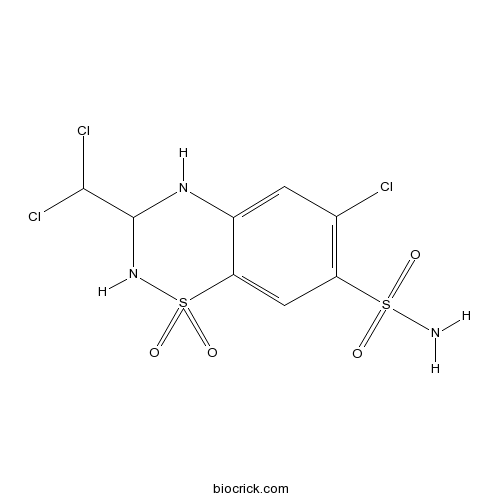
TrichlormethiazideCAS# 133-67-5 |
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Trovafloxacin mesylate
Catalog No.:BCC3931
CAS No.:147059-75-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 133-67-5 | SDF | Download SDF |
| PubChem ID | 5560 | Appearance | Powder |
| Formula | C8H8Cl3N3O4S2 | M.Wt | 380.66 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 150 mg/mL (394.05 mM; Need ultrasonic) | ||
| Chemical Name | 6-chloro-3-(dichloromethyl)-1,1-dioxo-3,4-dihydro-2H-1$l^{6},2,4-benzothiadiazine-7-sulfonamide | ||
| SMILES | C1=C2C(=CC(=C1Cl)S(=O)(=O)N)S(=O)(=O)NC(N2)C(Cl)Cl | ||
| Standard InChIKey | LMJSLTNSBFUCMU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H8Cl3N3O4S2/c9-3-1-4-6(2-5(3)19(12,15)16)20(17,18)14-8(13-4)7(10)11/h1-2,7-8,13-14H,(H2,12,15,16) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Trichlormethiazide Dilution Calculator

Trichlormethiazide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.627 mL | 13.1351 mL | 26.2702 mL | 52.5403 mL | 65.6754 mL |
| 5 mM | 0.5254 mL | 2.627 mL | 5.254 mL | 10.5081 mL | 13.1351 mL |
| 10 mM | 0.2627 mL | 1.3135 mL | 2.627 mL | 5.254 mL | 6.5675 mL |
| 50 mM | 0.0525 mL | 0.2627 mL | 0.5254 mL | 1.0508 mL | 1.3135 mL |
| 100 mM | 0.0263 mL | 0.1314 mL | 0.2627 mL | 0.5254 mL | 0.6568 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Trichlormethiazide is a thiazide diuretic with properties similar to those of hydrochlorothiazide.
- 3-Indolebutyric acid (IBA)
Catalog No.:BCC6491
CAS No.:133-32-4
- Asarinin
Catalog No.:BCN2769
CAS No.:133-05-1
- (-)-Asarinin
Catalog No.:BCN2290
CAS No.:133-04-0
- Fmoc-Lys(Fmoc)-OPfp
Catalog No.:BCC3522
CAS No.:132990-14-8
- Mequindox
Catalog No.:BCC9021
CAS No.:13297-17-1
- Macrocarpal A
Catalog No.:BCN6178
CAS No.:132951-90-7
- Rifampin
Catalog No.:BCC4839
CAS No.:13292-46-1
- 9-Hydroxy-13E-labden-15-oic acid
Catalog No.:BCN6177
CAS No.:132915-47-0
- Ramosetron Hydrochloride
Catalog No.:BCC5272
CAS No.:132907-72-3
- Bis(4-hydroxy-3,5-dimethylphenyl) sulfone
Catalog No.:BCC8884
CAS No.:13288-70-5
- HOE-S 785026
Catalog No.:BCC1633
CAS No.:132869-83-1
- Lercanidipine hydrochloride
Catalog No.:BCC5238
CAS No.:132866-11-6
- 13-Epijhanol
Catalog No.:BCN4713
CAS No.:133005-15-9
- (+)-SK&F 10047 hydrochloride
Catalog No.:BCC6928
CAS No.:133005-41-1
- Ethacrynic acid - d5
Catalog No.:BCC7987
CAS No.:1330052-59-9
- LY 225910
Catalog No.:BCC6891
CAS No.:133040-77-4
- GF 109203X
Catalog No.:BCC3704
CAS No.:133052-90-1
- Go 6983
Catalog No.:BCC3705
CAS No.:133053-19-7
- ZD 7288
Catalog No.:BCC6884
CAS No.:133059-99-1
- BIM 23052
Catalog No.:BCC5945
CAS No.:133073-82-2
- Fmoc-D-Lys(Boc)-OPfp
Catalog No.:BCC3527
CAS No.:133083-36-0
- Crassifoline methine
Catalog No.:BCN1793
CAS No.:133084-00-1
- 3PO
Catalog No.:BCC5616
CAS No.:13309-08-5
- Darifenacin
Catalog No.:BCC1516
CAS No.:133099-04-4
Comparison of Azelnidipine and Trichlormethiazide in Japanese Type 2 Diabetic Patients with Hypertension: The COAT Randomized Controlled Trial.[Pubmed:25938807]
PLoS One. 2015 May 4;10(5):e0125519.
OBJECTIVE: This study compared the efficacy and safety of azelnidipine with that of Trichlormethiazide in Japanese type 2 diabetic patients with hypertension. METHODS: In a multicenter, open-label trial, 240 patients with adequately controlled diabetes (HbA1c /= 130 mmHg or diastolic blood pressure [dBP] >/= 80 mmHg) who were being treated with olmesartan were enrolled. Participants were randomly assigned to an azelnidipine group or a Trichlormethiazide group and were followed up for 48 weeks. Main outcome measure was the difference in the change in HbA1c levels from the baseline values at 48 weeks between these two groups. RESULTS: Of the 240 subjects that were enrolled, 209 subjects (azelnidipine group: 103 patients, Trichlormethiazide group: 106 patients) completed this trial. At 48 weeks, the following changes were observed in the azelnidipine and Trichlormethiazide groups, respectively: HbA1c levels, 0.19 +/- 0.52% and 0.19 +/- 0.54%; sBP/dBP, -10.7 +/- 9.6/-6.6 +/- 6.6 mmHg and -7.1 +/- 7.7/-3.3 +/- 6.1 mmHg (P < 0.001 for both sBP and dBP). In both groups, dizziness (12 patients [11.7%] and 16 patients [15.1%]) and edema (16 patients [15.5%] and 7 patients [6.6%], P = 0.047) were observed during the 48-week follow-up period. CONCLUSIONS: Azelnidipine was more effective for controlling blood pressure than Trichlormethiazide in Japanese type 2 diabetes patients, whereas Trichlormethiazide was more effective for reducing albuminuria than azelnidipine. Both of these agents, however, similarly exacerbated glycemic control in type 2 diabetic patients with hypertension. TRIAL REGISTRATION: UMIN 000006081.
Comparison of spironolactone and trichlormethiazide as add-on therapy to renin-angiotensin blockade for reduction of albuminuria in diabetic patients.[Pubmed:24843672]
J Diabetes Investig. 2013 May 6;4(3):316-9.
To compare the efficacy of spironolactone and Trichlormethiazide, as add-on therapy to renin-angiotensin system (RAS) blockade, for reduction of albuminuria in diabetic patients with chronic kidney disease (CKD), we conducted this randomized, open-labeled, parallel-group, active-controlled, per-protocol-design study. Type 2 diabetic patients receiving an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker, with persistent albuminuria (>/=100 mg/g creatinine) were randomly assigned to either spironolactone (25 mg/day) or Trichlormethiazide (2 mg/day). The primary outcome was the change in albuminuria at 24 weeks of treatment. In patients who completed 24 weeks of treatment with spironolactone (n = 18) and Trichlormethiazide (n = 15), albuminuria decreased significantly by -57.6 +/- 21.3% (SD) (P < 0.001) and -48.4 +/- 27.1% (P < 0.001), respectively. There was no significant difference in the change in albuminuria between groups (P = 0.270). This pilot study suggests add-on therapy with spironolactone or Trichlormethiazide to RAS blockade may be comparably beneficial to reducing albuminuria in type 2 diabetic patients. This trial was registered with UMIN-CTR (no. UMIN000008914).
Olmesartan with azelnidipine versus with trichlormethiazide on home blood pressure variability in patients with type II diabetes mellitus.[Pubmed:28089902]
J Am Soc Hypertens. 2017 Mar;11(3):140-147.
The aim of the present study was to compare the effects of olmesartan combined with azelnidipine versus olmesartan combined with Trichlormethiazide, on home blood pressure (BP) and pressure variability in type II diabetes mellitus patients using home BP telemonitoring system. We performed an open-label cross-over pilot study of 28 patients with type II diabetes mellitus. Patients received combination treatment with either olmesartan 20 mg plus azelnidipine 16 mg or olmesartan 20 mg plus Trichlormethiazide 1 mg for more than 6 weeks each in a cross-over method. The coefficient of morning systolic BP variability in the olmesartan plus azelnidipine group was significantly lower than that in the olmesartan plus Trichlormethiazide group (6.4 +/- 1.9 vs. 7.5 +/- 2.6, P = .004). There were no significant differences in mean morning systolic BP between the two groups. Using home BP telemonitoring for hypertensive patients with type II diabetes, this study revealed for the first time that the olmesartan with azelnidipine combination is superior to the olmesartan with Trichlormethiazide combination in reducing home BP variability.
Identification of trichlormethiazide as a Mdr1a/b gene expression enhancer via a dual secretion-based promoter assay.[Pubmed:25692026]
Pharmacol Res Perspect. 2015 Feb;3(1):e00109.
Transporters of the ATP-binding cassette (ABC) family such as MDR1 play a pivotal role in persistence of brain homeostasis by contributing to the strict permeability properties of the blood-brain barrier. This barrier on one hand compromises treatment of central nervous system diseases by restricting access of drugs; on the other hand, an impaired or altered function of barrier building cells has been described in neurological disorders. The latter might contribute to increased vulnerability of the brain under pathological conditions or even enforce pathogenesis. Here, we present a novel approach for a systematic examination of drug impact on Mdr1 gene expression by establishing a dual reporter gene assay for the murine upstream core promoters of Mdr1a and b. We validated the time-resolved assay in comparison with single reporter gene constructs and applied it to analyze effects of a Food and Drug Administration (FDA)-approved drug library consisting of 627 substances. The chemo-preventive synthetic dithiolethione oltipraz was reidentified with our assay as an already known inducer of Mdr1 gene expression. Together with two newly characterized modifiers - gemcitabine and Trichlormethiazide - we prove our findings in a blood-brain barrier culture model as well as in wild-type and Mdr1 knockout mice. In sum, we could demonstrate that our dual reporter gene assay delivers results, which also persist in the living animal and consequently is applicable for further analysis and prediction of Mdr1 regulation in vivo.


