(-)-AsarininCAS# 133-04-0 |
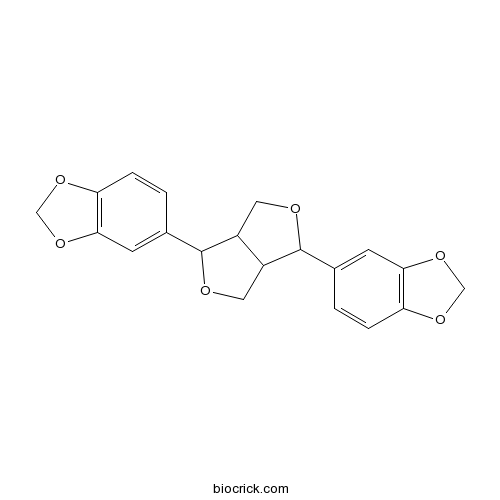
- Asarinin
Catalog No.:BCN2769
CAS No.:133-05-1
- Sesamin
Catalog No.:BCN4123
CAS No.:607-80-7
- (-)-Sesamin
Catalog No.:BCN9084
CAS No.:13079-95-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 133-04-0 | SDF | Download SDF |
| PubChem ID | 5204 | Appearance | White powder |
| Formula | C20H18O6 | M.Wt | 354.35 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Synonyms | Desgeranyloxyarmatumin; (-)-Episesamin; Xanthoxylin S | ||
| Solubility | Soluble in chloroform | ||
| Chemical Name | 5-[3-(1,3-benzodioxol-5-yl)-1,3,3a,4,6,6a-hexahydrofuro[3,4-c]furan-6-yl]-1,3-benzodioxole | ||
| SMILES | C1C2C(COC2C3=CC4=C(C=C3)OCO4)C(O1)C5=CC6=C(C=C5)OCO6 | ||
| Standard InChIKey | PEYUIKBAABKQKQ-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Asarinin, a mammalian lignan precursor, has immunosuppression activity in vitro. Asarinin can decrease peripheral blood concentration of interleukin (IL)-12 and inhibit the expression of Toll-like receptor 4 (TLR4) and chemokine (C-X-C motif) receptor 3 (CXCR3), which means asarinin may have a role on TLR4 pathway and produced prolongation of allograft heart survival. |
| Targets | IL Receptor | IFN-γ | CXCR | TLR |
| In vitro | Experimental study of the effects of Asarinin on immunosuppression activity in vitro.[Reference: WebLink]Chinese Journal of Cardiology, 2003, 31(6):444-7.To test the extract of Asarum heterotropides called Asarinin in the immunosuppression activity in vitro and compared with cyclosporine A(CsA).
|
| In vivo | The effect of Asarinin on Toll-like pathway in rats after cardiac allograft implantation.[Pubmed: 25769604]Transplant Proc. 2015 Mar;47(2):545-8.The objective of this study was to study the mechanism of the anti-rejection effect of Asarinin in rats that underwent cardiac allograft implantation.
|
| Kinase Assay | Inhibition of Δ5-desaturase in polyunsaturated fatty acid biosynthesis by (−)-asarinin and (−)-epiasarinin.[Reference: WebLink]Phytochemistry, 1992, 31(3):757-60.
|
| Structure Identification | Acta Crystallogr C. 2013 Jan;69(Pt 1):87-9.(±)-Asarinin.[Pubmed: 23282922]Asarinin, C(20)H(18)O(6), was isolated as a racemate from the shrub Zanthoxylum alatum.
Food Chem., 2011, 124(3):895-9.A new mammalian lignan precursor, asarinin.[Reference: WebLink]Enterolactone (ENL) and enterodiol are mammalian lignans.
|

(-)-Asarinin Dilution Calculator

(-)-Asarinin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8221 mL | 14.1103 mL | 28.2207 mL | 56.4414 mL | 70.5517 mL |
| 5 mM | 0.5644 mL | 2.8221 mL | 5.6441 mL | 11.2883 mL | 14.1103 mL |
| 10 mM | 0.2822 mL | 1.411 mL | 2.8221 mL | 5.6441 mL | 7.0552 mL |
| 50 mM | 0.0564 mL | 0.2822 mL | 0.5644 mL | 1.1288 mL | 1.411 mL |
| 100 mM | 0.0282 mL | 0.1411 mL | 0.2822 mL | 0.5644 mL | 0.7055 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Fmoc-Lys(Fmoc)-OPfp
Catalog No.:BCC3522
CAS No.:132990-14-8
- Mequindox
Catalog No.:BCC9021
CAS No.:13297-17-1
- Macrocarpal A
Catalog No.:BCN6178
CAS No.:132951-90-7
- Rifampin
Catalog No.:BCC4839
CAS No.:13292-46-1
- 9-Hydroxy-13E-labden-15-oic acid
Catalog No.:BCN6177
CAS No.:132915-47-0
- Ramosetron Hydrochloride
Catalog No.:BCC5272
CAS No.:132907-72-3
- Bis(4-hydroxy-3,5-dimethylphenyl) sulfone
Catalog No.:BCC8884
CAS No.:13288-70-5
- HOE-S 785026
Catalog No.:BCC1633
CAS No.:132869-83-1
- Lercanidipine hydrochloride
Catalog No.:BCC5238
CAS No.:132866-11-6
- PD 81723
Catalog No.:BCC7032
CAS No.:132861-87-1
- Terchebulin
Catalog No.:BCN3264
CAS No.:132854-40-1
- YS-49
Catalog No.:BCC2067
CAS No.:132836-42-1
- Asarinin
Catalog No.:BCN2769
CAS No.:133-05-1
- 3-Indolebutyric acid (IBA)
Catalog No.:BCC6491
CAS No.:133-32-4
- Trichlormethiazide
Catalog No.:BCC4872
CAS No.:133-67-5
- 13-Epijhanol
Catalog No.:BCN4713
CAS No.:133005-15-9
- (+)-SK&F 10047 hydrochloride
Catalog No.:BCC6928
CAS No.:133005-41-1
- Ethacrynic acid - d5
Catalog No.:BCC7987
CAS No.:1330052-59-9
- LY 225910
Catalog No.:BCC6891
CAS No.:133040-77-4
- GF 109203X
Catalog No.:BCC3704
CAS No.:133052-90-1
- Go 6983
Catalog No.:BCC3705
CAS No.:133053-19-7
- ZD 7288
Catalog No.:BCC6884
CAS No.:133059-99-1
- BIM 23052
Catalog No.:BCC5945
CAS No.:133073-82-2
- Fmoc-D-Lys(Boc)-OPfp
Catalog No.:BCC3527
CAS No.:133083-36-0
The effect of Asarinin on Toll-like pathway in rats after cardiac allograft implantation.[Pubmed:25769604]
Transplant Proc. 2015 Mar;47(2):545-8.
OBJECTIVE: The objective of this study was to study the mechanism of the anti-rejection effect of Asarinin in rats that underwent cardiac allograft implantation. METHODS: Hearts from Wistar rats were transplanted into the abdominal cavity of Sprague Dawley rats (SD rats) 64 SD rats received either cyclosporin A (CsA), Asarinin, or demi-dose of cyclosporine A and Asarinin through oral administration. On the seventh day post-transplantation, the expression of Toll-like receptor 4 (TLR4), chemokine (C-X-C motif) receptor 3 (CXCR3) in myocardium, and the level of interleukin (IL)-12 in the peripheral blood were analyzed 7 days after transplantation. RESULTS: The survival time in 3 groups (CsA group, Asarinin group, and semi-dose CsA group) prolonged (P < .01), the microscope myocardial histopathology in 3 groups (CsA group, Asarinin group and semi-dose CsA group) relieved, the expression of TLR4 and CXCR3 in 3 groups was significantly decreased (P < .01) when compared with the control group. The level of IL-12 decreased remarkably (P < .05) in the 3 groups when compared with the control group. CONCLUSIONS: The combined data suggested that Asarinin decreased peripheral blood concentration of IL-12 and inhibited the expression of TLR4 and CXCR3, which means Asarinin may have a role on TLR4 pathway and produced prolongation of allograft heart survival.
(+/-)-Asarinin.[Pubmed:23282922]
Acta Crystallogr C. 2013 Jan;69(Pt 1):87-9.
Asarinin, C(20)H(18)O(6), was isolated as a racemate from the shrub Zanthoxylum alatum. Both forms of the enantiomerically pure substance, (+)- and (-)-Asarinin, have been the subject of a total of five previous structure determinations that are essentially identical except for the absolute stereochemistry. However, there seems to be some confusion in the literature concerning these structure determinations of asarinin and also those of its stereoisomer sesamin. The molecular structure of racemic asarinin differs from that of the pure enantiomers in the orientation of one ring system. In the packing of the racemate, molecules are linked by C-H...O interactions to form ribbons parallel to [101].


