3POPFKFB3 inhibitor; antiangiogenic CAS# 13309-08-5 |
- Pyridostatin
Catalog No.:BCC1875
CAS No.:1085412-37-8
- 360A iodide
Catalog No.:BCC1308
CAS No.:737763-37-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13309-08-5 | SDF | Download SDF |
| PubChem ID | 5720233 | Appearance | Powder |
| Formula | C13H10N2O | M.Wt | 210.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
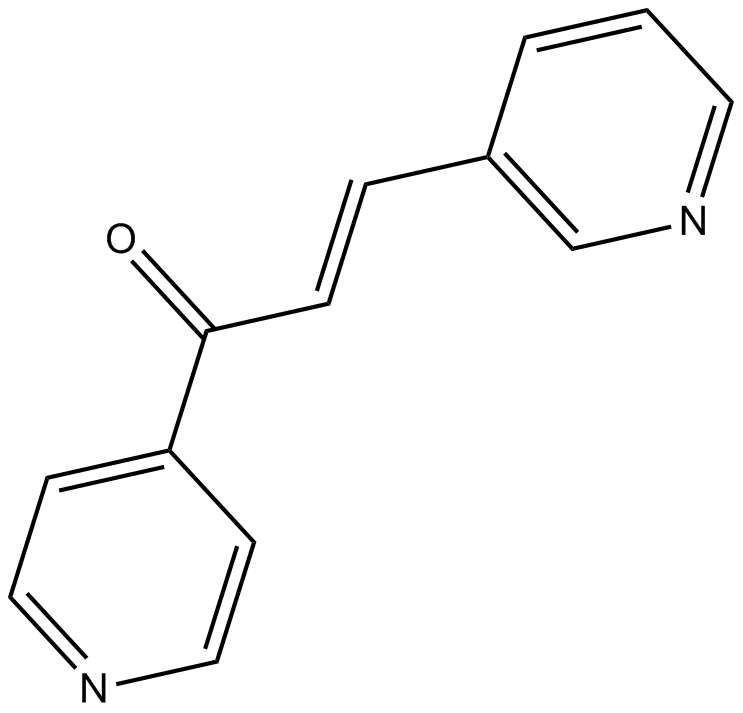
| Chemical Name | (E)-3-pyridin-3-yl-1-pyridin-4-ylprop-2-en-1-one | ||
| SMILES | C1=CC(=CN=C1)C=CC(=O)C2=CC=NC=C2 | ||
| Standard InChIKey | UOWGYMNWMDNSTL-ONEGZZNKSA-N | ||
| Standard InChI | InChI=1S/C13H10N2O/c16-13(12-5-8-14-9-6-12)4-3-11-2-1-7-15-10-11/h1-10H/b4-3+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Originally reported to inhibit PFKFB3 (IC50 = 25 μM). More recent reports failed to demonstrate activity in a PFKFB3 kinase assay. Reduces glycolytic flux and suppresses glucose uptake. Inhibits endothelial cell proliferation and causes G2/M cell cycle arrest in vitro. Attenuates vessel sprouting and tumor growth in vivo. Amplifies the antiangiogenic effect of VEGFR blockade. |

3PO Dilution Calculator

3PO Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.7567 mL | 23.7835 mL | 47.567 mL | 95.1339 mL | 118.9174 mL |
| 5 mM | 0.9513 mL | 4.7567 mL | 9.5134 mL | 19.0268 mL | 23.7835 mL |
| 10 mM | 0.4757 mL | 2.3783 mL | 4.7567 mL | 9.5134 mL | 11.8917 mL |
| 50 mM | 0.0951 mL | 0.4757 mL | 0.9513 mL | 1.9027 mL | 2.3783 mL |
| 100 mM | 0.0476 mL | 0.2378 mL | 0.4757 mL | 0.9513 mL | 1.1892 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Crassifoline methine
Catalog No.:BCN1793
CAS No.:133084-00-1
- Fmoc-D-Lys(Boc)-OPfp
Catalog No.:BCC3527
CAS No.:133083-36-0
- BIM 23052
Catalog No.:BCC5945
CAS No.:133073-82-2
- ZD 7288
Catalog No.:BCC6884
CAS No.:133059-99-1
- Go 6983
Catalog No.:BCC3705
CAS No.:133053-19-7
- GF 109203X
Catalog No.:BCC3704
CAS No.:133052-90-1
- LY 225910
Catalog No.:BCC6891
CAS No.:133040-77-4
- Ethacrynic acid - d5
Catalog No.:BCC7987
CAS No.:1330052-59-9
- (+)-SK&F 10047 hydrochloride
Catalog No.:BCC6928
CAS No.:133005-41-1
- 13-Epijhanol
Catalog No.:BCN4713
CAS No.:133005-15-9
- Trichlormethiazide
Catalog No.:BCC4872
CAS No.:133-67-5
- 3-Indolebutyric acid (IBA)
Catalog No.:BCC6491
CAS No.:133-32-4
- Darifenacin
Catalog No.:BCC1516
CAS No.:133099-04-4
- Darifenacin HBr
Catalog No.:BCC4567
CAS No.:133099-07-7
- Flutamide
Catalog No.:BCC4364
CAS No.:13311-84-7
- Epimedin B1
Catalog No.:BCN8199
CAS No.:133137-58-3
- GR 73632
Catalog No.:BCC5801
CAS No.:133156-06-6
- Oxypeucedan hydrate
Catalog No.:BCN8372
CAS No.:133164-11-1
- Fmoc-Cit-OH
Catalog No.:BCC3180
CAS No.:133174-15-9
- Fmoc-Thr(Trt)-OH
Catalog No.:BCC3554
CAS No.:133180-01-5
- PI3k-delta inhibitor 1
Catalog No.:BCC1861
CAS No.:1332075-63-4
- AM 92016 hydrochloride
Catalog No.:BCC6825
CAS No.:133229-11-5
- Heliangin
Catalog No.:BCN6487
CAS No.:13323-48-3
- 4-O-Methylbutein
Catalog No.:BCN6677
CAS No.:13323-67-6
Impact of the cellular prion protein on amyloid-beta and 3PO-tau processing.[Pubmed:24028865]
J Alzheimers Dis. 2014;38(3):551-65.
Previous studies indicate an important role for the cellular prion protein (PrP(C)) in the development of Alzheimer's disease (AD) pathology. In the present study, we analyzed the involvement of PrP(C) in different pathological mechanisms underlying AD: the processing of the amyloid-beta protein precursor (AbetaPP) and its interaction with AbetaPP, tau, and different phosphorylated forms of the tau protein (p-tau). The effect of PrP(C) on tau expression was investigated in various cellular compartments using a HEK293 cell model expressing a tau mutant (3PO-tau) or wild type (WT)-tau. We could show that PrP(C) reduces AbetaPP cleavage, leading to decreased levels of Abeta40 and sAbetaPP without changing the protein expression of AbetaPP, beta-secretase, or gamma-secretase. Tau and its phosphorylated forms were identified as interactions partners for PrP(C), raising the question as to whether PrP(C) might also be involved in tau pathology. Overexpression of PrP(C) in PRNP and 3PO-tau transfected cells resulted in a reduction of 3PO-tau and p-tau as well as a decrease of 3PO-tau-related toxicity. In addition, we used the transgenic PrP(C) knockout (Prnp0/0) mouse line to study the dynamics of tau phosphorylation, an important pathological hallmark in the pathogenesis of AD in vivo. There, an effect of PrP(C) on tau expression could be observed under oxidative stress conditions but not during aging. In summary, we provide further evidence for interactions of PrP(C) with proteins that are known to be the key players in AD pathogenesis. We identified tau and its phosphorylated forms as potential PrP-interactors and report a novel protective function of PrP(C) in AD-like tau pathology.
3PO, a novel nonviral gene delivery system using engineered Ad5 penton proteins.[Pubmed:11420644]
Gene Ther. 2001 May;8(10):795-803.
This study describes the development of 3PO, a nonviral, protein-based gene delivery vector which utilizes the highly evolved cell-binding, cell-entry and intracellular transport functions of the adenovirus serotype 5 (Ad5) capsid penton protein. A penton fusion protein containing a polylysine sequence was produced by recombinant methods and tested for gene delivery capability. As the protein itself is known to bind integrins through a conserved consensus motif, the penton inherently possesses the ability to bind and enter cells through receptor-mediated internalization. The ability to lyse the cellular endosome encapsulating internalized receptors is also attributed to the penton. The recombinant protein gains the additional function of DNA binding and transport with the appendage of a polylysine motif. This protein retains the ability to form pentamers and mediates delivery of a reporter gene to cultured cells. Interference by oligopeptides bearing the integrin binding motif suggests that delivery is mediated specifically through integrin receptor binding and internalization. The addition of protamine to penton-DNA complexes allows gene delivery in the presence of serum.
Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis.[Pubmed:24332967]
Cell Metab. 2014 Jan 7;19(1):37-48.
Strategies targeting pathological angiogenesis have focused primarily on blocking vascular endothelial growth factor (VEGF), but resistance and insufficient efficacy limit their success, mandating alternative antiangiogenic strategies. We recently provided genetic evidence that the glycolytic activator phosphofructokinase-2/fructose-2,6-bisphosphatase 3 (PFKFB3) promotes vessel formation but did not explore the antiangiogenic therapeutic potential of PFKFB3 blockade. Here, we show that blockade of PFKFB3 by the small molecule 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO) reduced vessel sprouting in endothelial cell (EC) spheroids, zebrafish embryos, and the postnatal mouse retina by inhibiting EC proliferation and migration. 3PO also suppressed vascular hyperbranching induced by inhibition of Notch or VEGF receptor 1 (VEGFR1) and amplified the antiangiogenic effect of VEGF blockade. Although 3PO reduced glycolysis only partially and transiently in vivo, this sufficed to decrease pathological neovascularization in ocular and inflammatory models. These insights may offer therapeutic antiangiogenic opportunities.
Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth.[Pubmed:18202014]
Mol Cancer Ther. 2008 Jan;7(1):110-20.
6-phosphofructo-1-kinase, a rate-limiting enzyme of glycolysis, is activated in neoplastic cells by fructose-2,6-bisphosphate (Fru-2,6-BP), a product of four 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase isozymes (PFKFB1-4). The inducible PFKFB3 isozyme is constitutively expressed by neoplastic cells and required for the high glycolytic rate and anchorage-independent growth of ras-transformed cells. We report herein the computational identification of a small-molecule inhibitor of PFKFB3, 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO), which suppresses glycolytic flux and is cytostatic to neoplastic cells. 3PO inhibits recombinant PFKFB3 activity, suppresses glucose uptake, and decreases the intracellular concentration of Fru-2,6-BP, lactate, ATP, NAD+, and NADH. 3PO markedly attenuates the proliferation of several human malignant hematopoietic and adenocarcinoma cell lines (IC50, 1.4-24 micromol/L) and is selectively cytostatic to ras-transformed human bronchial epithelial cells relative to normal human bronchial epithelial cells. The PFKFB3 enzyme is an essential molecular target of 3PO because transformed cells are rendered resistant to 3PO by ectopic expression of PFKFB3 and sensitive to 3PO by heterozygotic genomic deletion of PFKFB3. Importantly, i.p. administration of 3PO (0.07 mg/g) to tumor-bearing mice markedly reduces the intracellular concentration of Fru-2,6-BP, glucose uptake, and growth of established tumors in vivo. Taken together, these data support the clinical development of 3PO and other PFKFB3 inhibitors as chemotherapeutic agents.


