TolrestatCAS# 82964-04-3 |
- Resiquimod (R-848)
Catalog No.:BCC4073
CAS No.:144875-48-9
- TAK-242 S enantiomer
Catalog No.:BCC1978
CAS No.:243984-10-3
- TAK-242
Catalog No.:BCC1977
CAS No.:243984-11-4
- Imiquimod maleate
Catalog No.:BCC4197
CAS No.:896106-16-4
- Imiquimod hydrochloride
Catalog No.:BCC4196
CAS No.:99011-78-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 82964-04-3 | SDF | Download SDF |
| PubChem ID | 53359 | Appearance | Powder |
| Formula | C16H14F3NO3S | M.Wt | 357.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AY-27773 | ||
| Solubility | DMSO : ≥ 100 mg/mL (279.84 mM) *"≥" means soluble, but saturation unknown. | ||
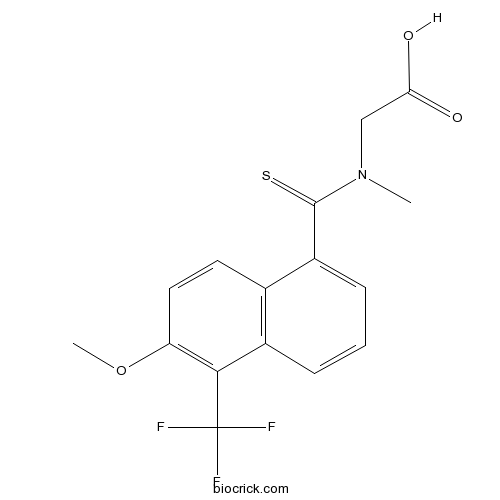
| Chemical Name | 2-[[6-methoxy-5-(trifluoromethyl)naphthalene-1-carbothioyl]-methylamino]acetic acid | ||
| SMILES | CN(CC(=O)O)C(=S)C1=CC=CC2=C1C=CC(=C2C(F)(F)F)OC | ||
| Standard InChIKey | LUBHDINQXIHVLS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H14F3NO3S/c1-20(8-13(21)22)15(24)11-5-3-4-10-9(11)6-7-12(23-2)14(10)16(17,18)19/h3-7H,8H2,1-2H3,(H,21,22) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tolrestat is a potent, orally active aldose reductase inhibitor with IC50 of 35 nM.In Vivo:Tolrestat (1.8 mg/kg per day) causes a reversal to normal RBC sorbitol levels diabetic rats[1]. In 21-day diabetic rats, the estimated ID in the sciatic nerve and lenses is 4.8 and about 20 for tolrestat, and 1.7 and 2.2 for (±)sorbinil,
respectively[2]. Either tolrestat or sorbinil inhibits tissue AR activity but does not significantly affect plasma lipoprotein levels, or affect the body weight of the mice or their general health. Accumulation of cholesterol-rich foam cells is significantly increased in aortic roots of tolrestat-fed mice[3]. References: | |||||

Tolrestat Dilution Calculator

Tolrestat Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7984 mL | 13.9919 mL | 27.9838 mL | 55.9675 mL | 69.9594 mL |
| 5 mM | 0.5597 mL | 2.7984 mL | 5.5968 mL | 11.1935 mL | 13.9919 mL |
| 10 mM | 0.2798 mL | 1.3992 mL | 2.7984 mL | 5.5968 mL | 6.9959 mL |
| 50 mM | 0.056 mL | 0.2798 mL | 0.5597 mL | 1.1194 mL | 1.3992 mL |
| 100 mM | 0.028 mL | 0.1399 mL | 0.2798 mL | 0.5597 mL | 0.6996 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tolrestat(AY-27773) is a potent, orally active aldose reductase inhibitor with IC50 value of 35 nM.
- 4-O-Galloylbergenin
Catalog No.:BCN6643
CAS No.:82958-45-0
- 11-O-Galloylbergenin
Catalog No.:BCN6637
CAS No.:82958-44-9
- Nafamostat Mesylate(FUT-175)
Catalog No.:BCC1228
CAS No.:82956-11-4
- CI 898 trihydrochloride
Catalog No.:BCC7248
CAS No.:82952-64-5
- 7,4'-Dihydroxy-8-methylflavan
Catalog No.:BCN6841
CAS No.:82925-55-1
- Fmoc-Osu
Catalog No.:BCC2804
CAS No.:82911-69-1
- 3-Oxo-24,25,26,27-tetranortirucall-7-en-23,21-olide
Catalog No.:BCN1338
CAS No.:828935-47-3
- (R)-(+)-Etomoxir sodium salt
Catalog No.:BCC7946
CAS No.:828934-41-4
- 2,3-Dihydroisoginkgetin
Catalog No.:BCN4035
CAS No.:828923-27-9
- Cyclo(Tyr-Leu)
Catalog No.:BCN2432
CAS No.:82863-65-8
- Echinacoside
Catalog No.:BCN4953
CAS No.:82854-37-3
- Pingpeimine B
Catalog No.:BCN8408
CAS No.:82851-52-3
- 4-Aminoantipyrine
Catalog No.:BCC8683
CAS No.:83-07-8
- Phenindione
Catalog No.:BCC4699
CAS No.:83-12-5
- 1-Indanone
Catalog No.:BCN2245
CAS No.:83-33-0
- Methylprednisolone
Catalog No.:BCC2256
CAS No.:83-43-2
- Deoxycholic acid
Catalog No.:BCN1288
CAS No.:83-44-3
- Beta-Sitosterol
Catalog No.:BCN1015
CAS No.:83-46-5
- Stigmasterol
Catalog No.:BCN4376
CAS No.:83-48-7
- Hyodeoxycholic acid
Catalog No.:BCN1287
CAS No.:83-49-8
- 5-Amino-1-naphthol
Catalog No.:BCC8729
CAS No.:83-55-6
- Theobromine
Catalog No.:BCN1227
CAS No.:83-67-0
- 2-Hydroxy-1,4-naphoquinone
Catalog No.:BCN8398
CAS No.:83-72-7
- Ibogaine
Catalog No.:BCN4378
CAS No.:83-74-9
Influence of tolrestat on the defective leukocyte-endothelial interaction in experimental diabetes.[Pubmed:10720648]
Eur J Pharmacol. 2000 Mar 10;391(1-2):163-74.
One of the most devastating secondary complications of diabetes is the blunted inflammatory response that becomes evident even in the very early stages of poorly controlled diabetes mellitus. While the etiology of this diminished response is not clearly understood, it has been linked to a decrease in the respiratory burst of neutrophils, as well as a decrease in microvessel response to inflammatory mediators and defective leukocyte-endothelial interactions. Using video microscopy to visualize vessels of the internal spermatic fascia, we have characterized leukocyte-endothelial interactions in alloxan-induced diabetic and in galactosemic rats by quantitating the number of leukocytes rolling along the venular endothelium and the number of leukocytes sticking to the vascular wall after topical application of zymosan-activated plasma or leukotriene B(4) (1 ng/ml), as well as after the application of a local irritant stimulus (carrageenan, 100 microg). We observed that while 33 days of alloxan-induced diabetes or 7 days of galactosemia had no effect on total or differential leukocyte counts and on the wall shear rate, both treatments significantly (P<0.001) reduced the number of leukocytes rolling along the venular endothelium by about 70% and the number of adhered leukocytes in postcapillary venules by 60%. These effects were not observed in diabetic and galactosemic animals treated with an aldose reductase inhibitor. The results suggest that impaired leukocyte-endothelial cell interactions are a consequence of an enhanced flux through the polyol pathway.
Effects of aminoguanidine and tolrestat on the development of ocular and renal structural changes in experimental diabetic rats.[Pubmed:11874446]
Diabetes Obes Metab. 2002 Jan;4(1):75-9.
Studies that researched the role of aminoguanidine and tolestat in the prevention of diabetic retinopathy and nephropathy resulted in conflicting data. We investigated the effects of these agents in the prevention of ocular and renal changes in streptozotocin (STZ)-induced diabetic rats. Diabetes was induced by intravenous injection of STZ in 30 rats. Ten rats that were not given STZ served as non-diabetic control (Group 1). Ten STZ-diabetic rats that were not given any treatment served as diabetic control (Group 2). Groups 3 and 4 were composed of STZ-induced diabetic rats (10 each) that were given Tolrestat and aminoguanidine respectively. Eyes and kidneys were examined at the 24th week under electronmicroscopy. Cataract was observed in all six of the surviving rats in Groups 2 and 4, and in one of 6 surviving rats in group 3. Cataract development was lower in Group 3 than Groups 2 and 4. All retinal samples obtained from group 2 demonstrated a number of structural abnormalities, whereas there were no significant ultrastructural changes in groups 3 and 4. Groups 2 and 3 demonstrated mesangial proliferation and expansion, diffuse glomerular basement membrane (GBM) thickening, and focal GBM thickening in the bulb form. Group 4 demonstrated a normally appearing mesangial space, minimal diffuse but no focal GBM thickening. The urinary albumin excretion (UAE) was lower in Group 4 than the other groups. In conclusion, our results suggest that aminoguanidine may be an important agent for the prevention of renal changes, whereas Tolrestat may be effective for the prevention of ocular changes in diabetes mellitus.
Hydrogen bonding interactions between aldose reductase complexed with NADP(H) and inhibitor tolrestat studied by molecular dynamics simulations and binding assay.[Pubmed:12604217]
Chem Biol Interact. 2003 Feb 1;143-144:307-16.
Molecular dynamics simulations and binding affinity studies have been carried out in order to probe the effect of the charge state of His110 and cofactor NADPH on the binding affinity of the potent inhibitor Tolrestat to aldose reductase (ALR2) complexed with either NADPH or NADP(+). Molecular dynamics simulations of ALR2-NADP(+)-Tolrestat indicate that the carboxylate group of Tolrestat forms a hydrogen bond with Tyr48 and His110 of ALR2 regardless of the charge state of His110. In the case of ALR2-NADPH-Tolrestat, the H-bonding pattern is significantly different from that of ALR2-NADP(+)-Tolrestat, in that Tyr48 does not H-bond to Tolrestat. The binding affinity of Tolrestat to ALR2 complexed with either NADPH or NADP(+) is comparable and pH-dependent. Based on the H-bonding interactions seen in computer simulations, it is proposed that the cationic moiety at the active site of ALR2-NADP(+) and ALR2-NADPH that interacts with the carboxylate of Tolrestat is NADP(+) and His110, respectively. The residue that gives rise to the pH-dependent binding of Tolrestat to ALR2-NADP(+) and ALR2-NADPH has been identified as Tyr48 and His110, respectively.
Correction of nerve conduction and endoneurial blood flow deficits by the aldose reductase inhibitor, tolrestat, in diabetic rats.[Pubmed:10959252]
J Peripher Nerv Syst. 1998;3(3):217-23.
Increased activation of the first half of the polyol pathway, the conversion of glucose to sorbitol by aldose reductase, has been implicated in aldose reductase inhibitor-preventable neurochemical changes that may contribute to the aetiology of diabetic neuropathy. Tolrestat has been used as a standard aldose reductase inhibitor to dissect out polyol pathway-dependent mechanisms in many experimental studies; however, doubt has been cast upon its ability to prevent nerve conduction velocity deficits in diabetic rats. Nerve dysfunction has also been linked to abnormal endoneurial blood flow and oxygenation via increased vasa nervorum polyol pathway flux. The aim of this study was to test whether Tolrestat could correct sciatic conduction velocity and perfusion defects in diabetic rats. Sciatic motor conduction velocity, 21% reduced by 1 month of streptozotocin-induced diabetes, was corrected by 23% and 84% with 1 month of Tolrestat treatment at doses of 7 and 35 mg/kg/day respectively. Endoneurial blood flow, 44-52% reduced by untreated diabetes, was within the nondiabetic range with high-dose Tolrestat treatment and the flow deficit was 39% corrected by the low dose. Sciatic sorbitol and fructose concentrations were approximately 13-fold and approximately 4-fold elevated by untreated diabetes. This was 32-50% attenuated by low-dose Tolrestat and sorbitol and fructose content was suppressed below the nondiabetic level by high dose treatment. A 58% nerve myo-inositol deficit was partially (32%) corrected by high-dose Tolrestat treatment. We conclude that Tolrestat restores defective conduction and blood flow in diabetic rats and is a good pharmacological tool for studies on polyol pathway effects in peripheral nerve.


