Nafamostat Mesylate(FUT-175)Serine protease inhibitor CAS# 82956-11-4 |
- Leupeptin, Microbial
Catalog No.:BCC1217
CAS No.:103476-89-7
- BCX 1470
Catalog No.:BCC1413
CAS No.:217099-43-9
- BCX 1470 methanesulfonate
Catalog No.:BCC1414
CAS No.:217099-44-0
- PMSF
Catalog No.:BCC1229
CAS No.:329-98-6
- Nafamostat hydrochloride
Catalog No.:BCC4188
CAS No.:80251-32-7
- Nafamostat
Catalog No.:BCC4187
CAS No.:81525-10-2
Quality Control & MSDS
Number of papers citing our products

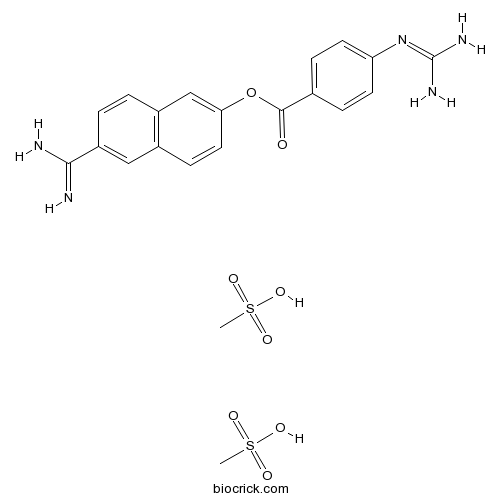
Chemical structure
3D structure
| Cas No. | 82956-11-4 | SDF | Download SDF |
| PubChem ID | 5311180 | Appearance | Powder |
| Formula | C21H25N5O8S2 | M.Wt | 539.59 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | FUT-175 | ||
| Solubility | DMSO : 50 mg/mL (92.66 mM; Need ultrasonic) H2O : 33.33 mg/mL (61.77 mM; Need ultrasonic) | ||
| Chemical Name | 4-[(Aminoiminomethyl)amino]benzoic acid 6-(aminoiminomethyl)-2-naphthalenyl | ||
| SMILES | CS(=O)(=O)O.CS(=O)(=O)O.C1=CC(=CC=C1C(=O)OC2=CC3=C(C=C2)C=C(C=C3)C(=N)N)N=C(N)N | ||
| Standard InChIKey | SRXKIZXIRHMPFW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H17N5O2.2CH4O3S/c20-17(21)14-2-1-13-10-16(8-5-12(13)9-14)26-18(25)11-3-6-15(7-4-11)24-19(22)23;2*1-5(2,3)4/h1-10H,(H3,20,21)(H4,22,23,24);2*1H3,(H,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Broad spectrum serine protease inhibitor. Displays selectivity for human tryptase when used at relatively low concentrations. Reduces eosinophil infiltration, mast cell activation and airway responsiveness in a murine model of asthma. |
| Cell experiment [1]: | |
| Cell lines | The human pancreatic tumor cell lines PANC-1 |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 3 h; 160 μg/mL |
| Applications | In assessment of the NF- κB activation by ELISA, concentration of NF- κB p65 in the nuclear extracts of PANC-1 cells in combination group was statistically lower than those in oxaliplatin group (p<0.0001). Like nuclear NF-κB levels, phosphorylated IκBa levels by Western blot analysis in combination group were significantly lower than those in oxaliplatin group (p=0.037). In other words, FUT-175 inhibits oxaliplatin-induced NF- κB activation by suppressing IκBa phosphorylation in vitro. |
| Animal experiment [1]: | |
| Animal models | Five-week-old male nude mice |
| Dosage form | 30 μg/g; thrice a week for 6 weeks; intraperitoneal injection |
| Application | A pancreatic cancer model was established by injection of PANC-1 cells (5×10-6cells) in 200 μM of PBS subcutaneously into the right side of the back of the animals. In vivo, the tumor growth in combination group (oxaliplatin and nafamostat mesilate) was significantly slower than that of oxaliplatin group (p<0.0001). Tumor volume in combination group was significantly smaller than that of oxaliplatin group (p=0.048). |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Gocho T, Uwagawa T, Furukawa K, et al. Combination chemotherapy of serine protease inhibitor nafamostat mesilate with oxaliplatin targeting NF-κB activation for pancreatic cancer[J]. Cancer letters, 2013, 333(1): 89-95. | |

Nafamostat Mesylate(FUT-175) Dilution Calculator

Nafamostat Mesylate(FUT-175) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8533 mL | 9.2663 mL | 18.5326 mL | 37.0652 mL | 46.3315 mL |
| 5 mM | 0.3707 mL | 1.8533 mL | 3.7065 mL | 7.413 mL | 9.2663 mL |
| 10 mM | 0.1853 mL | 0.9266 mL | 1.8533 mL | 3.7065 mL | 4.6331 mL |
| 50 mM | 0.0371 mL | 0.1853 mL | 0.3707 mL | 0.7413 mL | 0.9266 mL |
| 100 mM | 0.0185 mL | 0.0927 mL | 0.1853 mL | 0.3707 mL | 0.4633 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
In previous studies, nafamostat mesylate inhibited NF-KB activation and induced apoptosis in pancreatic cancer cells. Nafamostat mesylate is to be evaluated for those effects in gallbladder cancer cells pre-treated with gemcitabine.
Abstract
Nafamostate prolonged hemofilter lifespan from 10.2 h to 19.8 h without increasing RBC transfusion in CRRT patients at high risk of bleeding.
Abstract
Nafamostat mesilate is a serine protease inhibitor that exhibits complementaing-modifying effects and anticoagulant properties and has been largely used in Aisa to control inflammation.
Abstract
Pancreatitis is the most common complication of RRCP.
Abstract
Nafamostat mesilate, a serine protease inhibitor, has been assessed for efficacy and safety in CRRT patients.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Nafamostat mesylate, previously known as FUT-175, is an inhibitor of serine protease that inhibits a variety of serine proteases, including trypsin and several proteases in the coagulation cascade. Although it was originally developed as an inhibitor of complements, Nafamostat mesylate has been widely used for the treatment of inflammation (such as acute pancreatitis) and disseminated intravascular coagulation (DIC). Nafamostat mesylate exhibits extremely potent inhibition against human tryptase as well as tryptase-catalyzed hydrolysis of Boc-Phe-Ser-Arg-MCA with inhibition constant Kivalue of 95.3 pM. Besides its protease-inhibiting activity, nafamostat mesylate, in a recent study, displayed its antimicrobial activity by dose-dependently inhibiting the proliferation of chlamydial in vitro.
Reference
Robert D Inman and Basil Chiu. Nafamostat mesylate, a serine protease inhibitor, demonstrates novel antimicrobial properties and effectiveness in Chlamydia-induced arthritis. Arthritis Rsearch & Therapy 2012, 13:R150
Shuji Mori, Yoshinori Itoh, Ryoko Shinohata, Toshiaki Sendo, Ryozo Oishi and Masahiro Nishibori. Nafamostat mesilate is an extremely potent inhibitor of human tryptase. J Pharmacol Sci 92, 420-423 (2003)
- CI 898 trihydrochloride
Catalog No.:BCC7248
CAS No.:82952-64-5
- 7,4'-Dihydroxy-8-methylflavan
Catalog No.:BCN6841
CAS No.:82925-55-1
- Fmoc-Osu
Catalog No.:BCC2804
CAS No.:82911-69-1
- 3-Oxo-24,25,26,27-tetranortirucall-7-en-23,21-olide
Catalog No.:BCN1338
CAS No.:828935-47-3
- (R)-(+)-Etomoxir sodium salt
Catalog No.:BCC7946
CAS No.:828934-41-4
- 2,3-Dihydroisoginkgetin
Catalog No.:BCN4035
CAS No.:828923-27-9
- Cyclo(Tyr-Leu)
Catalog No.:BCN2432
CAS No.:82863-65-8
- Echinacoside
Catalog No.:BCN4953
CAS No.:82854-37-3
- Pingpeimine B
Catalog No.:BCN8408
CAS No.:82851-52-3
- Pingpeimine A
Catalog No.:BCN8407
CAS No.:82841-67-6
- Perindopril
Catalog No.:BCC4223
CAS No.:82834-16-0
- cGMP Dependent Kinase Inhibitor Peptide
Catalog No.:BCC8084
CAS No.:82801-73-8
- 11-O-Galloylbergenin
Catalog No.:BCN6637
CAS No.:82958-44-9
- 4-O-Galloylbergenin
Catalog No.:BCN6643
CAS No.:82958-45-0
- Tolrestat
Catalog No.:BCC4084
CAS No.:82964-04-3
- 4-Aminoantipyrine
Catalog No.:BCC8683
CAS No.:83-07-8
- Phenindione
Catalog No.:BCC4699
CAS No.:83-12-5
- 1-Indanone
Catalog No.:BCN2245
CAS No.:83-33-0
- Methylprednisolone
Catalog No.:BCC2256
CAS No.:83-43-2
- Deoxycholic acid
Catalog No.:BCN1288
CAS No.:83-44-3
- Beta-Sitosterol
Catalog No.:BCN1015
CAS No.:83-46-5
- Stigmasterol
Catalog No.:BCN4376
CAS No.:83-48-7
- Hyodeoxycholic acid
Catalog No.:BCN1287
CAS No.:83-49-8
- 5-Amino-1-naphthol
Catalog No.:BCC8729
CAS No.:83-55-6
Partial filling affinity capillary electrophoresis as a useful tool for fragment-based drug discovery: A proof of concept on thrombin.[Pubmed:28843566]
Anal Chim Acta. 2017 Sep 1;984:211-222.
With the emergence of more challenging targets, a relatively new approach, fragment-based drug discovery (FBDD), proved its efficacy and gained increasing importance in the pharmaceutical industry. FBDD identifies low molecular-weight (MW) ligands (fragments) that bind to biologically important macromolecules, then a structure-guided fragment growing or merging approach is performed, contributing to the quality of the lead. However, to select the appropriate fragment to be evolved, sensitive analytical screening methods must be used to measure the affinity in the muM or even mM range. In this particular context, we developed a robust and selective partial filling affinity CE (ACE) method for the direct binding screening of a small fragment library in order to identify new thrombin inhibitors. To demonstrate the accuracy of our assay, the complex dissociation constants of three known thrombin inhibitors, namely benzamidine, p-aminobenzamidine and nafamostat were determined and found to be in good concordance with the previously reported values. Finally, the screening of a small library was performed and demonstrated the high discriminatory power of our method towards weak binders compared to classical spectrophotometric activity assay, proving the interest of our method in the context of FBDD.
Adsorption of Nafamostat Mesilate on AN69ST Membranes: A Single-Center Retrospective and In Vitro Study.[Pubmed:28960755]
Ther Apher Dial. 2017 Dec;21(6):620-627.
We examined whether AN69ST (acrylonitrile and methallyl sulfonate copolymer) membranes adsorb nafamostat mesilate. This study retrospectively analyzed 87 continuous hemodiafiltration sessions in vivo. We divided the continuous hemodiafiltration sessions into AN69ST and non-AN69ST groups using the nafamostat mesilate dose and activated clotting time as indicators of nafamostat mesilate adsorption onto the membrane. Furthermore, we studied the in vitro adsorption of nafamostat mesilate from nafamostat mesilate solutions onto four different hemodialysis membranes. This in vivo study shows that nafamostat mesilate doses were significantly higher, but activated clotting times were shorter (P < 0.001) in the AN69ST group than in the non-AN69ST group. These results suggest that AN69ST adsorbs nafamostat mesilate. Further, the in vitro experiments show that nafamostat mesilate adsorbs AN69ST on membranes significantly more than the other membranes tested. These in vitro and clinical findings provide evidence that AN69ST may adsorb nafamostat mesilate.
Clinical study of blood purification therapy in critical care in Japan: results from the survey research of the Japan Society for Blood Purification in Critical Care in 2013.[Pubmed:28600615]
J Artif Organs. 2017 Sep;20(3):244-251.
To clarify the clinical status of blood purification therapy (BPT) in critical care in Japan, we conducted a cohort study using data from a nationwide registry of the Japan Society for Blood Purification in Critical Care in 2013. We enrolled 2227 patients treated with BPT (female, 39.1%; mean age, 65.5 +/- 12.1 years) in the intensive care units of 43 facilities. Patient characteristics, modes of BPT, and survival rate for each disease were investigated. In total, BPT was performed 3053 times. Continuous renal replacement therapy (CRRT) (57.9%) was the most common mode of BPT, followed by intermittent renal replacement therapy (20.2%) and direct hemoperfusion with the polymyxin B-immobilized fiber column (PMX-DHP) (11.5%). Nafamostat mesilate (84.9%) was most frequently used as the anticoagulant. The 28-day survival rate was 56.8% in all patients. The most common mode for acute kidney injury (AKI) and multiple organ failure was CRRT, while PMX-DHP and CRRT were most common for sepsis. There was no significant difference in survival rates among AKI stages 1-3. Survival rate (38.3%) was significantly lower in patients with acute lung injury (ALI) than in those with multiple organ failure (41.8%) and those with sepsis (46.6%). Multivariate regression analysis revealed that the APACHE II score and the presence of acute ALI and acute hepatic failure were significantly associated with death. This large-scale cohort study showed the clinical status of BPT in Japan. Further investigations are required to clarify the efficacy of BPT for critically ill patients.
AN69ST membranes adsorb nafamostat mesylate and affect the management of anticoagulant therapy: a retrospective study.[Pubmed:28729905]
J Intensive Care. 2017 Jul 18;5:46.
BACKGROUND: In Japan, nafamostat mesylate (NM) is frequently used as an anticoagulant during continuous renal replacement therapy (CRRT). The dialyzer membrane AN69ST has been reported to adsorb NM and affect the management of anticoagulant therapy. However, the adsorbed amount has not yet been quantitatively assessed. Therefore, in this study, we evaluated the pre- and post-hemofilter prolongation of the activated clotting time (ACT) in patients with AN69ST and PS membranes. We also measured the adsorption of NM in three types of CRRT membranes using an experimental model. METHODS: In a study of patients who underwent CRRT using AN69ST or PS membranes in 2015 at the Advanced Emergency and Critical Care Center, Okayama University Hospital, pre- and post-hemofilter ACT measurements were extracted retrospectively, and the difference was calculated. In addition, AN69ST (sepXiris100), PS (HEMOFEEL SHG-1.0), and PMMA membranes (HEMOFEEL CH-1.0N) were used in an in vitro model of a dialysis circuit, and the concentrations of NM were measured in pre- and post-hemofilter membranes and filtrates. RESULTS: The ACT difference was significantly lower in the group using AN69ST membranes (p < 0.01). In the in vitro model (n = 4) with adsorption and filtration, the post-hemofilter and filtrate concentrations of NM in AN69ST membranes were significantly lower than those in the PS and PMMA membranes (p < 0.01). The NM adsorption clearance of the AN69ST membrane was significantly higher than that of the PS and PMMA membranes. CONCLUSIONS: The AN69ST membrane had higher NM adsorption than the PS and PMMA membranes. This may have resulted in the lower ACT difference in patients undergoing CRRT using the AN69ST membrane than in patients undergoing CRRT using PS or PMMA membranes.
Serine protease inhibitors nafamostat mesilate and gabexate mesilate attenuate allergen-induced airway inflammation and eosinophilia in a murine model of asthma.[Pubmed:16815145]
J Allergy Clin Immunol. 2006 Jul;118(1):105-12.
BACKGROUND: Serine proteases such as mast cell tryptase and certain allergens are important in the pathogenesis of allergic inflammation of asthma. OBJECTIVE: We sought to investigate the effects of serine protease inhibitors nafamostat mesilate (FUT), gabexate mesilate (FOY), and ulinastatin (UTI) on airway inflammation in a mouse model of allergic asthma. METHODS: BALB/c mice were sensitized to Dermatophagoides pteronyssinus (Der p) and intratracheally challenged with Der p (0.5 mg/mL). Therapeutic doses of FUT (0.0625 mg/kg), FOY (20 mg/kg), and UTI (10,000 U/kg) were intra-peritoneally injected into 3 corresponding sensitized mice during the sensitization phase (protocol 1) or 24 hours after allergen challenge (protocol 2). RESULTS: Both FUT-treated and FOY-treated sensitized mice had reduced mast cells activation, airway hyperresponsiveness, attenuated eosinophils infiltrations, and decreased Der p-induced IL-4 and TNF-alpha, but increased IL-12 cytokine production in bronchoalveolar lavage fluid compared with nontreated mice. Furthermore, FUT treatment downregulated the expression of IL-1beta, TNF-alpha, IL-6, eotaxin, inducible NO synthase, CD86, and nuclear factor-kappaB activation, but enhanced the expression of IL-12 and IL-10 in Der p-stimulated alveolar macrophages. UTI-treated mice have no significant change of the aforementioned measurements compared with nontreated sensitized mice. CONCLUSION: Nafamostat mesilate and FOY exerting the therapeutic effect in allergen-induced airway inflammation was a result not only of their inhibitory action in the early phase of mast cells activation but also of immunoregulatory function in the late phase of allergic inflammation. Such properties of FUT and FOY might be a potential therapeutic approach for asthma. CLINICAL IMPLICATIONS: The clinical used of serine protease inhibitors FUT and FOY may also have implications for treating airway inflammation of asthma.
Nafamostat mesilate is an extremely potent inhibitor of human tryptase.[Pubmed:12939527]
J Pharmacol Sci. 2003 Aug;92(4):420-3.
Previously, nafamostat mesilate was found to be a potent inhibitor of human tryptase. In present study, we performed a kinetic study to determine its K(i) value for tryptase and compared it with that of gabexate mesilate. Nafamostat mesilate inhibited human tryptase in a competitive manner. The apparent K(i) value was estimated to be 95.3 pM, which was 1000 times lower than that of gabexate mesilate (95.1 nM). These results strongly indicated that nafamostat mesilate is an extremely potent inhibitor of tryptase and suggested that some of its beneficial effects in the treatment of clinical status may be due to tryptase inhibition.
A potent tryptase inhibitor nafamostat mesilate dramatically suppressed pulmonary dysfunction induced in rats by a radiographic contrast medium.[Pubmed:12642398]
Br J Pharmacol. 2003 Mar;138(5):959-67.
(1) Intravenous injection of ioxaglate (4 g iodine kg(-1)), an iodinated radiographic contrast medium, caused a marked protein extravasation, pulmonary oedema and a decrease in the arterial partial oxygen pressure in rats. (2) All of these reactions to ioxaglate were reversed by the pretreatment with gabexate mesilate (10 and 50 mg kg(-1), 5 min prior to injection) or nafamostat mesilate (3 and 10 mg kg(-1)), in which the inhibition was complete after injection of nafamostat mesilate (10 mg kg(-1)). (3) Both gabexate mesilate and nafamostat mesilate inhibited the activity of purified human lung tryptase, although the latter compound was far more potent than the former. (4) Ioxaglate enhanced the nafamostat-sensitive protease activity in the extracellular fluid of rat peritoneal mast cell suspensions. (5) Tryptase enhanced the permeability of protein through the monolayer of cultured human pulmonary arterial endothelial cells. Ioxaglate, when applied in combination with rat peritoneal mast cells, also produced the endothelial barrier dysfunction. These effects of tryptase and ioxaglate were reversed by nafamostat mesilate. (6) Consistent with these findings, immunofluorescence morphological analysis revealed that tryptase or ioxaglate in combination with mast cells increased actin stress fibre formation while decreasing VE-cadherin immunoreactivity. Both of these actions of tryptase and ioxaglate were reversed by nafamostat mesilate. (7) These findings suggest that tryptase liberated from mast cells plays a crucial role in the ioxaglate-induced pulmonary dysfunction. In this respect, nafamostat mesilate may become a useful agent for the cure or prevention of severe adverse reactions to radiographic contrast media.


