4-AminoantipyrineCAS# 83-07-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 83-07-8 | SDF | Download SDF |
| PubChem ID | 2151 | Appearance | Powder |
| Formula | C11H13N3O | M.Wt | 203 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Ampyrone | ||
| Solubility | DMSO : ≥ 50 mg/mL (246.01 mM) H2O : 50 mg/mL (246.01 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
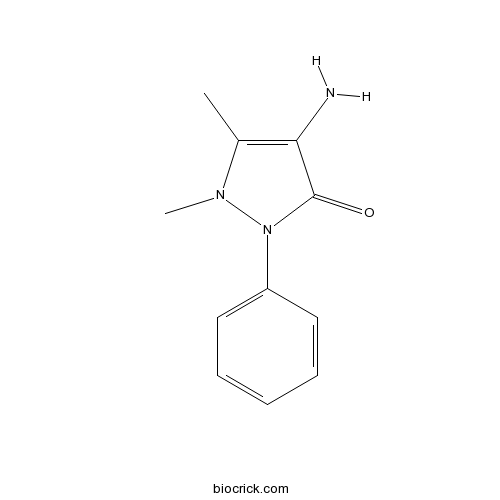
| Chemical Name | 4-amino-1,5-dimethyl-2-phenylpyrazol-3-one | ||
| SMILES | CC1=C(C(=O)N(N1C)C2=CC=CC=C2)N | ||
| Standard InChIKey | RLFWWDJHLFCNIJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H13N3O/c1-8-10(12)11(15)14(13(8)2)9-6-4-3-5-7-9/h3-7H,12H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

4-Aminoantipyrine Dilution Calculator

4-Aminoantipyrine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.9261 mL | 24.6305 mL | 49.2611 mL | 98.5222 mL | 123.1527 mL |
| 5 mM | 0.9852 mL | 4.9261 mL | 9.8522 mL | 19.7044 mL | 24.6305 mL |
| 10 mM | 0.4926 mL | 2.4631 mL | 4.9261 mL | 9.8522 mL | 12.3153 mL |
| 50 mM | 0.0985 mL | 0.4926 mL | 0.9852 mL | 1.9704 mL | 2.4631 mL |
| 100 mM | 0.0493 mL | 0.2463 mL | 0.4926 mL | 0.9852 mL | 1.2315 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
4-Aminoantipyrine is a reagent for glucose determination in the presence of peroxidase and phenol.
- Tolrestat
Catalog No.:BCC4084
CAS No.:82964-04-3
- 4-O-Galloylbergenin
Catalog No.:BCN6643
CAS No.:82958-45-0
- 11-O-Galloylbergenin
Catalog No.:BCN6637
CAS No.:82958-44-9
- Nafamostat Mesylate(FUT-175)
Catalog No.:BCC1228
CAS No.:82956-11-4
- CI 898 trihydrochloride
Catalog No.:BCC7248
CAS No.:82952-64-5
- 7,4'-Dihydroxy-8-methylflavan
Catalog No.:BCN6841
CAS No.:82925-55-1
- Fmoc-Osu
Catalog No.:BCC2804
CAS No.:82911-69-1
- 3-Oxo-24,25,26,27-tetranortirucall-7-en-23,21-olide
Catalog No.:BCN1338
CAS No.:828935-47-3
- (R)-(+)-Etomoxir sodium salt
Catalog No.:BCC7946
CAS No.:828934-41-4
- 2,3-Dihydroisoginkgetin
Catalog No.:BCN4035
CAS No.:828923-27-9
- Cyclo(Tyr-Leu)
Catalog No.:BCN2432
CAS No.:82863-65-8
- Echinacoside
Catalog No.:BCN4953
CAS No.:82854-37-3
- Phenindione
Catalog No.:BCC4699
CAS No.:83-12-5
- 1-Indanone
Catalog No.:BCN2245
CAS No.:83-33-0
- Methylprednisolone
Catalog No.:BCC2256
CAS No.:83-43-2
- Deoxycholic acid
Catalog No.:BCN1288
CAS No.:83-44-3
- Beta-Sitosterol
Catalog No.:BCN1015
CAS No.:83-46-5
- Stigmasterol
Catalog No.:BCN4376
CAS No.:83-48-7
- Hyodeoxycholic acid
Catalog No.:BCN1287
CAS No.:83-49-8
- 5-Amino-1-naphthol
Catalog No.:BCC8729
CAS No.:83-55-6
- Theobromine
Catalog No.:BCN1227
CAS No.:83-67-0
- 2-Hydroxy-1,4-naphoquinone
Catalog No.:BCN8398
CAS No.:83-72-7
- Ibogaine
Catalog No.:BCN4378
CAS No.:83-74-9
- Rotenone
Catalog No.:BCN5412
CAS No.:83-79-4
Directed aryl sulfotransferase evolution toward improved sulfation stoichiometry on the example of catechols.[Pubmed:30830250]
Appl Microbiol Biotechnol. 2019 Mar 4. pii: 10.1007/s00253-019-09688-0.
Sulfation is an important way for detoxifying xenobiotics and endobiotics including catechols. Enzymatic sulfation occurs usually with high chemo- and/or regioselectivity under mild reaction conditions. In this study, a two-step p-NPS-4-AAP screening system for laboratory evolution of aryl sulfotransferase B (ASTB) was developed in 96-well microtiter plates to improve the sulfate transfer efficiency toward catechols. Increased transfer efficiency and improved sulfation stoichiometry are achieved through the two-step screening procedure in a one-pot reaction. In the first step, the p-NPS assay is used (detection of the colorimetric by-product, p-nitrophenol) to determine the apparent ASTB activity. The sulfated product, 3-chlorocatechol-1-monosulfate, is quantified by the 4-Aminoantipyrine (4-AAP) assay in the second step. Comparison of product formation to p-NPS consumption ensures successful directed evolution campaigns of ASTB. Optimization yielded a coefficient of variation below 15% for the two-step screening system (p-NPS-4-AAP). In total, 1760 clones from an ASTB-SeSaM library were screened toward the improved sulfation activity of 3-chlorocatechol. The turnover number (kcat = 41 +/- 2 s(-1)) and catalytic efficiency (kcat/KM = 0.41 muM(-1) s(-1)) of the final variant ASTB-M5 were improved 2.4- and 2.3-fold compared with ASTB-WT. HPLC analysis confirmed the improved sulfate stoichiometry of ASTB-M5 with a conversion of 58% (ASTB-WT 29%; two-fold improvement). Mass spectrometry (MS) and nuclear magnetic resonance spectroscopy (NMR) confirmed the chemo- and regioselectivity, which yielded exclusively 3-chlorocatechol-1-monosulfate. For all five additionally investigated catechols, the variant ASTB-M5 achieved an improved kcat value of up to 4.5-fold and sulfate transfer efficiency was also increased (up to 2.3-fold).
Chemically stable inhibitors of 14-3-3 protein-protein interactions derived from BV02.[Pubmed:30727786]
J Enzyme Inhib Med Chem. 2019 Dec;34(1):657-664.
14-3-3 are regulatory proteins that through protein-protein interactions (PPI) with numerous binding partners could be involved in several human diseases, including cancer, neurodegenerative disorders, and pathogens infections. Following our research interest in the development of 14-3-3 PPI inhibitors, here we exploited the privileged 4-Aminoantipyrine scaffold in the design and synthesis of some derivatives endowed with antiproliferative activity against K-562 cells, and capable of binding to recombinant 14-3-3sigma as evidenced by NMR spectroscopy. The binding mode was further explored by molecular modelling, while coupling confocal microscopy with intensitometric analysis showed that compound 1 was able to promote the nuclear translocation of c-Abl at low micromolar concentrations. Overall, 1 is chemically stable compared to parent 14-3-3 PPI inhibitors, and thus emerged as a confirmed hit for further development.
A rapid throughput assay for screening (R)-2-(4-hydroxyphenoxy)propionic acid producing microbes.[Pubmed:30703447]
J Microbiol Methods. 2019 Mar;158:44-51.
(R)-2-(4-hydroxyphenoxy)propionic acid ((R)-HPOPA) is an important intermediate for the synthesis of optically pure aryloxyphenoxypropionic acid herbicides. Regioselective hydroxylation of (R)-2-phenoxypropionic acid ((R)-POPA) by microbes is one of the most useful methods for the industrial production of (R)-HPOPA. In this study, we designed and optimized a rapid throughput assay for screening (R)-HPOPA producing bacterial/fungal strains which can regioselectively hydroxylate (R)-POPA. (R)-HPOPA could react with 4-Aminoantipyrine (4-AAP) in the presence of potassium hexacyanoferrate (K3[Fe(CN)6]) to form indoxyl antipyrine, an orange-red chromophore, that can easily spectrophotometrically be determined at 550nm. During the verification of the assay we observed an average recovery rate of between 97.3% and 104.5%. Apart from the rapid throughput, no obvious differences in detection (R)-HPOPA in the culture broth samples were found between our rapid throughput multiplate assay and a high-performance liquid chromatography method. Our optimized assay method is simple, rapid and accurate with high repeatability. It has the potential for high throughput screening (about 3000-5000 samples/day) of the (R)-HPOPA producing strains.
Non-immunological toxicological mechanisms of metamizole-associated neutropenia in HL60 cells.[Pubmed:30653950]
Biochem Pharmacol. 2019 Jan 14;163:345-356.
Metamizole is an analgesic and antipyretic, but can cause neutropenia and agranulocytosis. We investigated the toxicity of the metabolites N-methyl-4-Aminoantipyrine (MAA), 4-Aminoantipyrine (AA), N-formyl-4-Aminoantipyrine (FAA) and N-acetyl-4-Aminoantipyrine (AAA) on neutrophil granulocytes and on HL60 cells (granulocyte precursor cell line). MAA, FAA, AA, and AAA (up to 100microM) alone were not toxic for HL60 cells or granulocytes. In the presence of the myeloperoxidase substrate H2O2, MAA reduced cytotoxicity for HL60 cells at low concentrations (<50microM), but increased cytotoxicity at 100microM H2O2. Neutrophil granulocytes were resistant to H2O2 and MAA. Fe(2+) and Fe(3+) were not toxic to HL60 cells, irrespective of the presence of H2O2 and MAA. Similarly, MAA did not increase the toxicity of lactoferrin, hemoglobin or methemoglobin for HL60 cells. Hemin (hemoglobin degradation product containing a porphyrin ring and Fe(3+)) was toxic on HL60 cells and cytotoxicity was increased by MAA. EDTA, N-acetylcystein and glutathione prevented the toxicity of hemin and hemin/MAA. The absorption spectrum of hemin changed concentration-dependently after addition of MAA, suggesting an interaction between Fe(3+) and MAA. NMR revealed the formation of a stable MAA reaction product with a reaction pathway involving the formation of an electrophilic intermediate. In conclusion, MAA, the principle metabolite of metamizole, increased cytotoxicity of hemin by a reaction involving the formation of an electrophilic metabolite. Accordingly, cytotoxicity of MAA/hemin could be prevented by the iron chelator EDTA and by the electron donors NAC and glutathione. Situations with increased production of hemin may represent a risk factor for metamizole-associated granulocytopenia.
A novel aluminum-sensitive fluorescent chemosensor based on 4-aminoantipyrine: An experimental and theoretical study.[Pubmed:30594851]
Spectrochim Acta A Mol Biomol Spectrosc. 2019 Apr 5;212:32-41.
A practical and an efficient Schiff base fluorescent chemosensor, salicylidene-4-aminoantipyrinyl-4-aminophenol (A2) has been synthesized through the condensation procedure of 1-phenyl-2,3-dimethyl-4-(N-2-hydroxybenzylidene)-3-pyrazoline-5-one and 4-aminophenol. Compound A2 has displayed a considerable fluorescence enhancement with high selectivity and sensitivity toward Al(3+) ion and exhibited an emission band at 484nm, which contained a low detection limit (LOD) of 1.06x10(-7)M. In accordance to the experimental study, DFT, TDDFT calculations, and the enhancement of fluorescence intensity might be attributed to the inhibition of Photoinduced Electron Transfer (PET) along with the Excited-State Intramolecular Proton Transfer (ESIPT). As it has been specified by Job's plot and DFT calculations, the binding stoichiometries of A2 with Al(3+) are 1:1, while the association constant (Ka) of Al(3+) has been calculated and observed to be 2.67xx 10(5)M(-1). Furthermore, the binding behavior and sensing mechanism of A2 with Al(3+) have been confirmed through the experiments of (1)H NMR titration.
Development of colorimetric cholesterol detection kit using TPU nanofibre/cellulose acetate membrane.[Pubmed:30095412]
IET Nanobiotechnol. 2018 Aug;12(5):557-561.
In this study, the authors report a simple fabrication of thermoplastic polyurethane (TPU) nanofibres-based kit for cholesterol detection. TPU is a polymer that is highly elastic, resistant to microorganisms, abrasion and compatible with blood; thus, making it a natural selection as an immobilisation matrix for cholesterol oxidase (ChOx) enzyme. The nanofibre was fabricated by electrospinning process and was characterised using scanning electron microscopy and Fourier transform-infrared spectroscopy. ChOx was covalently immobilised on TPU nanofibre and cholesterol level/concentration was visually found using 4-Aminoantipyrine, a dye that reacts with H2O2 produced from the oxidation of cholesterol by ChOx and changes colour from yellow to red. The efficacy of the nanofibre to act as a detecting substrate was compared with cellulose acetate (CA) membrane, a well-documented enzyme immobilisation matrix. The optimisation of enzyme concentration and dye quantity were performed using standard ChOx spectrophotometric assay and the same was used in CA membrane and TPU nanofibre. The ChOx immobilised nanofibre showed good linear range from 2 to 10 mM with a lower detection limit of 2 mM and was highly stable compared to that of CA membrane. The enzyme immobilised nanofibre was further validated in serum samples.
N-demethylation of N-methyl-4-aminoantipyrine, the main metabolite of metamizole.[Pubmed:29746911]
Eur J Pharm Sci. 2018 Jul 30;120:172-180.
Metamizole is an old analgesic used frequently in some countries. Active metabolites of metamizole are the non-enzymatically generated N-methyl-4-Aminoantipyrine (4-MAA) and its demethylation product 4-Aminoantipyrine (4-AA). Previous studies suggested that 4-MAA demethylation can be performed by hepatic cytochrome P450 (CYP) 3A4, but the possible contribution of other CYPs remains unclear. Using human liver microsomes (HLM), liver homogenate and HepaRG cells, we could confirm 4-MAA demethylation by CYPs. Based on CYP induction (HepaRG cells) and CYP inhibition (HLM) we could identify CYP2B6, 2C8, 2C9 and 3A4 as major contributors to 4-MAA demethylation. The 4-MAA demethylation rate by HLM was 280pmol/mg protein/h, too low to account for in vivo 4-MAA demethylation in humans. Since peroxidases can perform N-demethylation, we investigated horseradish peroxidase and human myeloperoxidase (MPO). Horse radish peroxidase efficiently demethylated 4-MAA, depending on the hydrogen peroxide concentration. This was also true for MPO; this reaction was saturable with a Km of 22.5muM and a maximal velocity of 14nmol/min/mg protein. Calculation of the entire body MPO capacity revealed that the demethylation capacity by granulocyte/granulocyte precursors was approximately 600 times higher than the liver capacity and could account for 4-MAA demethylation in humans. 4-MAA demethylation could also be demonstrated in MPO-expressing granulocyte precursor cells (HL-60). In conclusion, 4-MAA can be demethylated in the liver by several CYPs, but hepatic metabolism cannot fully explain 4-MAA demethylation in humans. The current study suggests that the major part of 4-MAA is demethylated by circulating granulocytes and granulocyte precursors in bone marrow.
Degradation of 4-aminoantipyrine by electro-oxidation with a boron-doped diamond anode: Optimization by central composite design, oxidation products and toxicity.[Pubmed:29727934]
Sci Total Environ. 2018 Aug 1;631-632:1079-1088.
Electro-oxidation with electrogenerated H2O2 (EO-H2O2) was applied to treat acidic aqueous solutions of 4-Aminoantipyrine (4-AA), a persistent drug metabolite of dipyrone, in sulfate medium. Trials were made using a boron-doped diamond anode in the presence of H2O2 electrogenerated on site. A 2(4) central composite design (CCD) was employed to evaluate the effect of four independent variables, namely current density (j), pH, 4-AA concentration and electrolysis time, on the percentages of degradation and mineralization, as well as on mineralization current efficiency (MCE). Predicted responses agreed with observed values, showing linear trendlines with good R(2) and R(2)adj values. The degradation was optimum at j=77.5mAcm(-2), pH3.5 and 62.5mgL(-1) 4-AA, leading to 63% and 99% removal after 3 and 7min, respectively. For those solutions, the largest mineralization was found at j=77.5mAcm(-2), attaining 45% abatement at 175min. Low MCE values were obtained in all electrolyses. An initial route for 4-AA degradation is proposed based on one dimer and eleven aromatic and aliphatic intermediates detected in the treated solutions at pH3.5 by LC-MS. The initial 62.5mgL(-1) solution at pH3.5 presented acute toxicity on Artemia salina larvae, with LC50=13.6mgL(-1), being substantially reduced after 3 and 7min of EO-H2O2 at j=77.5mAcm(-2) due to the formation of less toxic derivatives.
Interference of carbidopa and other catechols with reactions catalyzed by peroxidases.[Pubmed:29649511]
Biochim Biophys Acta Gen Subj. 2018 Jul;1862(7):1626-1634.
BACKGROUND: A number of compounds, including ascorbic acid, catecholamines, flavonoids, p-diphenols and hydrazine derivatives have been reported to interfere with peroxidase-based medical diagnostic tests (Trinder reaction) but the mechanisms of these effects have not been fully elucidated. METHODS: Reactions of bovine myeloperoxidase with o-dianisidine, bovine lactoperoxidase with ABTS and horseradish peroxidase with 4-Aminoantipyrine/phenol in the presence of carbidopa, an anti-Parkinsonian drug, and other catechols, including l-dopa, were monitored spectrophotometrically and by measuring hydrogen peroxide consumption. RESULTS: Chromophore formation in all three enzyme/substrate systems was blocked in the presence of carbidopa and other catechols. However, the rates of hydrogen peroxide consumption were not much affected. Irreversible enzyme inhibition was also insignificant. CONCLUSIONS: Tested compounds reduced the oxidation products or intermediates of model substrates thus preventing chromophore formation. This interference may affect interpretation of results of diagnostic tests in samples from patients with Parkinson's disease treated with carbidopa and l-dopa. GENERAL SIGNIFICANCE: This mechanism allows prediction of interference in peroxidase-based diagnostic tests for other compounds, including drugs and natural products.
Assessment of genetic integrity, splenic phagocytosis and cell death potential of (Z)-4-((1,5-dimethyl-3-oxo-2-phenyl-2,3dihydro-1H-pyrazol-4-yl) amino)-4-oxobut-2-enoic acid and its effect when combined with commercial chemotherapeutics.[Pubmed:29473933]
Genet Mol Biol. 2018 Jan-Mar;41(1):154-166.
The increased incidence of cancer and its high treatment costs have encouraged the search for new compounds to be used in adjuvant therapies for this disease. This study discloses the synthesis of (Z)-4-((1,5-dimethyl-3-oxo-2-phenyl-2,3dihydro-1H-pyrazol-4-yl) amino)-4-oxobut-2-enoic acid (IR-01) and evaluates not only the action of this compound on genetic integrity, increase in splenic phagocytosis and induction of cell death but also its effects in combination with the commercial chemotherapeutic agents doxorubicin, cisplatin and cyclophosphamide. IR-01 was designed and synthesized based on two multifunctionalyzed structural fragments: 4-Aminoantipyrine, an active dipyrone metabolite, described as an antioxidant and anti-inflammatory agent; and the pharmacophore fragment 1,4-dioxo-2-butenyl, a cytotoxic agent. The results indicated that IR-01 is an effective chemoprotector because it can prevent clastogenic and/or aneugenic damage, has good potential to prevent genomic damage, can increase splenic phagocytosis and lymphocyte frequency and induces cell death. However, its use as an adjuvant in combination with chemotherapy is discouraged since IR-01 interferes in the effectiveness of the tested chemotherapeutic agents. This is a pioneer study as it demonstrates the chemopreventive effects of IR-01, which may be associated with the higher antioxidant activity of the precursor structure of 4-Aminoantipyrine over the effects of the 1,4-dioxo-2-butenyl fragment.
Detonation Nanodiamonds as a New Tool for Phenol Detection in Aqueous Medium.[Pubmed:29458597]
J Nanosci Nanotechnol. 2018 Aug 1;18(8):5448-5453.
This paper demonstrates the effectiveness of using detonation nanodiamonds (DNDs) for detecting phenol in aqueous medium. The study has shown that the catalytic effect of DNDs in the oxidative azo coupling reaction (phenol-4-Aminoantipyrine-hydrogen peroxide) is produced by trace amounts of iron and copper ions adsorbed on the surface of nanoparticles. The effectiveness of DNDs as a catalyst is determined by the amounts of these adsorbates and can be enhanced by a factor of two by additional adsorption of these ions onto the nanoparticles. A rise in the temperature of the DND-catalyzed azo coupling reaction leads to a considerable (4.5-fold) increase in the reaction product yield. DNDs used to detect phenol in aqueous medium enable a linear increase in the yield of the product of the azo coupling reaction at concentrations of the analyte of between 0.05 and 10 mug/ml. The study demonstrates that DNDs can be reused to detect phenol in water samples.


