TicagrelorP2Y12 receptor antagonist CAS# 274693-27-5 |
- 5-OMe-UDP trisodium salt
Catalog No.:BCC6153
CAS No.:1207530-98-0
- AR-C 66096 tetrasodium salt
Catalog No.:BCC6004
CAS No.:145782-74-7
- Prasugrel hydrochloride
Catalog No.:BCC4291
CAS No.:389574-19-0
- Prasugrel Maleic acid
Catalog No.:BCC4292
CAS No.:389574-20-3
- MRS 2578
Catalog No.:BCC4976
CAS No.:711019-86-2
- AZD1283
Catalog No.:BCC5370
CAS No.:919351-41-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 274693-27-5 | SDF | Download SDF |
| PubChem ID | 9871419 | Appearance | Powder |
| Formula | C23H28F2N6O4S | M.Wt | 522.57 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AR-C 126532XX; AZD6140 | ||
| Solubility | DMSO : ≥ 50 mg/mL (95.68 mM) *"≥" means soluble, but saturation unknown. | ||
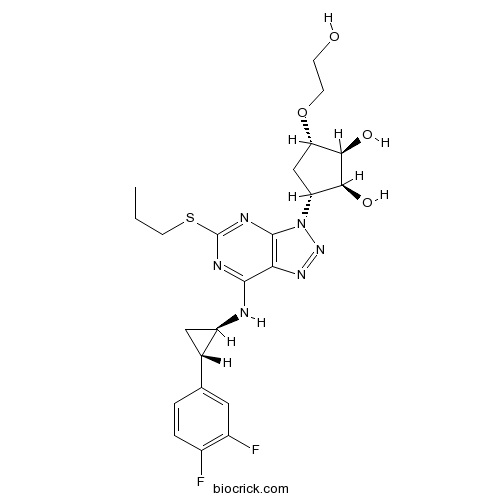
| Chemical Name | (1S,2S,3R,5S)-3-[7-[[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]amino]-5-propylsulfanyltriazolo[4,5-d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)cyclopentane-1,2-diol | ||
| SMILES | CCCSC1=NC2=C(C(=N1)NC3CC3C4=CC(=C(C=C4)F)F)N=NN2C5CC(C(C5O)O)OCCO | ||
| Standard InChIKey | OEKWJQXRCDYSHL-FNOIDJSQSA-N | ||
| Standard InChI | InChI=1S/C23H28F2N6O4S/c1-2-7-36-23-27-21(26-15-9-12(15)11-3-4-13(24)14(25)8-11)18-22(28-23)31(30-29-18)16-10-17(35-6-5-32)20(34)19(16)33/h3-4,8,12,15-17,19-20,32-34H,2,5-7,9-10H2,1H3,(H,26,27,28)/t12-,15+,16+,17-,19-,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ticagrelor (AZD6140) is a reversible oral P2Y12 receptor antagonist for the treatment of platelet aggregation.In Vitro:Ticagrelor promotes a greater inhibition of adenosine 5′-diphosphate (ADP)–induced Ca2+ release in ished platelets vs other P2Y12R antagonists. This additional effect of ticagrelor beyond P2Y12R antagonism is in part as a consequence of ticagrelor inhibiting the equilibrative nucleoside transporter 1 (ENT1) on platelets, leading to accumulation of extracellular adenosine and activation of Gs-coupled adenosine A2A receptors[1]. B16-F10 cells exhibit decreased interaction with platelets from ticagrelor-treated mice compared to saline-treated mice[2].In Vivo:In B16-F10 melanoma intravenous and intrasplenic metastasis models, mice treated with a clinical dose of ticagrelor (10 mg/kg) exhibits marked reductions in lung (84%) and liver (86%) metastases. Furthermore, ticagrelor treatment improves survival compared to saline-treated animals. A similar effect is observed in a 4T1 breast cancer model, with reductions in lung (55%) and bone marrow (87%) metastases following ticagrelor treatment[2]. Single oral administration of ticagrelor (1-10 mg/kg) causes dose-related inhibitory effect on platelet aggregation. Ticagrelor, at the highest dose (10 mg/kg) significantly inhibits platelet aggregation at 1 h after dosing and the peak inhibition is observed at 4 h after dosing[3]. References: | |||||

Ticagrelor Dilution Calculator

Ticagrelor Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9136 mL | 9.5681 mL | 19.1362 mL | 38.2724 mL | 47.8405 mL |
| 5 mM | 0.3827 mL | 1.9136 mL | 3.8272 mL | 7.6545 mL | 9.5681 mL |
| 10 mM | 0.1914 mL | 0.9568 mL | 1.9136 mL | 3.8272 mL | 4.784 mL |
| 50 mM | 0.0383 mL | 0.1914 mL | 0.3827 mL | 0.7654 mL | 0.9568 mL |
| 100 mM | 0.0191 mL | 0.0957 mL | 0.1914 mL | 0.3827 mL | 0.4784 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ticagrelor is a novel antagonist of the P2Y12 receptor [1].
Ticagrelor has been reported to inhibit the prothrombotic effects of ADP on the platelet by against the P2Y12 receptor. Ticagrelor has shown the complete inhibition of platelet aggregation ex vivo. In addition Ticagrelor has suggested a dose-dependent inhibition of platelet aggregation in human being. Apart from these, Ticagrelor has also demonstrated an orally, actively, reversibly binding antagonist. Unlike other inhibitors, Ticagrelor has also reported to inhibit P2Y12 receptor without metabolic transformation. Besides that, Ticagrelor is the first thienopyridine anti-platelet agent and mainly metabolized by CYP3A4 and CYP2C19 [1][2].
References:
[1] Zhou D1, Andersson TB, Grimm SW. In vitro evaluation of potential drug-drug interactions with ticagrelor: cytochrome P450 reaction phenotyping, inhibition, induction, and differential kinetics. Drug Metab Dispos. 2011 Apr;39(4):703-10.
[2] Li Y1, Landqvist C, Grimm SW. Disposition and metabolism of ticagrelor, a novel P2Y12 receptor antagonist, in mice, rats, and marmosets. Drug Metab Dispos. 2011 Sep;39(9):1555-67. doi: 10.1124/dmd.111.039669. Epub 2011 Jun 13.
- Neocryptotanshinone II
Catalog No.:BCN3138
CAS No.:27468-20-8
- Xanthohumol D
Catalog No.:BCN5162
CAS No.:274675-25-1
- 5-Methoxyisatin
Catalog No.:BCC8098
CAS No.:39755-95-8
- Coumarin 7
Catalog No.:BCC8920
CAS No.:27425-55-4
- Picroside I
Catalog No.:BCN6322
CAS No.:27409-30-9
- threo-Guaiacylglycerol
Catalog No.:BCN5161
CAS No.:27391-16-8
- 4'-Hydroxy-7-methoxyflavan
Catalog No.:BCN3497
CAS No.:27348-54-5
- VX-765
Catalog No.:BCC3648
CAS No.:273404-37-8
- Precyasterone
Catalog No.:BCN2754
CAS No.:27335-85-9
- 5-Formyl-2-furylboronic acid
Catalog No.:BCC8748
CAS No.:27329-70-0
- Tirapazamine
Catalog No.:BCC5184
CAS No.:27314-97-2
- ICI 63197
Catalog No.:BCC7188
CAS No.:27277-00-5
- LE 300
Catalog No.:BCC7148
CAS No.:274694-98-3
- 7-Acetoxy-4-methylcoumarin
Catalog No.:BCC8775
CAS No.:2747-05-9
- H-Leu-OtBu.HCl
Catalog No.:BCC2974
CAS No.:2748-02-9
- O-Benzyldauricine
Catalog No.:BCC8222
CAS No.:2748-99-4
- Cyclo(D-Val-L-Pro)
Catalog No.:BCN4015
CAS No.:27483-18-7
- trans-4-Aminocyclohexanol
Catalog No.:BCC9181
CAS No.:27489-62-9
- L-Ala-ol
Catalog No.:BCC2590
CAS No.:2749-11-3
- 2-O-Acetyltutin
Catalog No.:BCN5163
CAS No.:2749-28-2
- Vildagliptin (LAF-237)
Catalog No.:BCC2112
CAS No.:274901-16-5
- Protostemonine
Catalog No.:BCN8172
CAS No.:27495-40-5
- Physalin C
Catalog No.:BCN7918
CAS No.:27503-33-9
- O-Methylpallidine
Catalog No.:BCN3916
CAS No.:27510-33-4
Effect of ticagrelor on the serum level of hs-CRP, ESM-1 and short-term prognosis of patients with acute STEMI.[Pubmed:28352337]
Exp Ther Med. 2017 Feb;13(2):604-608.
The aim of the present study was to observe and investigate the changes in the serum level of high-sensitivity C-reactive protein (hs-CRP), the endothelial cell-specific molecule-1 (ESM-1) and short-term prognosis of patients with ST-segment elevation myocardial infarction (STEMI) treated by Ticagrelor. We enrolled 107 patients with acute STEMI who were admitted in the Department of Cardiology for the first time with occurrence of symptoms, and we successfully performed emergency operation of percutaneous coronary intervention. The patients were divided into two groups, 54 patients in the Ticagrelor group (treatment group) and 53 patients in the clopidogrel group (control group), according to the administration of Ticagrelor or clopidogrel in dual anti-platelet therapy. Then, we observed the changes at the time of admission, at 24 h, and 4th and 7th day after administration and investigated the correlations between them and the effect of Ticagrelor on the short-term prognosis of acute STEMI patients. Significant increases of the serum levels of hs-CRP and ESM-1 were seen in patients of the two groups 24 h after administration of drugs with statistically significant differences between the groups (P<0.05), and on the 4th and 7th day we found a downward trend with statistically significant differences (P<0.05). The level of ESM-1 enhanced the increase of hs-CRP, indicating there was a positive correlation between ESM-1 and hs-CRP (r=0.535, P<0.001). A comparison of the occurrence rates of ischemic outcome event, bleeding and overall adverse events between the two groups yielded no statistically significant difference (P>0.05). In conclusion, the present study demonstrates that Ticagrelor can reduce the prevalence of inflammatory reactions rapidly and stabilize the functions of vascular endothelium to improve the stability of atherosclerosis plaque and decrease the occurrence rate of thrombosis as well as ischemic outcome event without any obvious increase in the risk of bleeding. Thus, Ticagrelor should be recommended in clinical practices for the treatment of patients with STEMI.
The pharmacodynamics of low and standard doses of ticagrelor in patients with end stage renal disease on hemodialysis.[Pubmed:28342632]
Int J Cardiol. 2017 Jul 1;238:110-116.
BACKGROUND: Patients with end-stage renal disease (ESRD) on maintenance hemodialysis (HD) respond poorly to clopidogrel. We assessed the utility of low-dose Ticagrelor in ESRD patients on maintenance HD. METHODS: In this single-center, prospective, randomized pharmacodynamic study, 52 ESRD patients on HD were prescribed clopidogrel (300mg loading dose [LD], then 75mg daily), standard-dose Ticagrelor (180mg LD, then 90mg twice daily), or low-dose Ticagrelor (90mg LD, then 90mg daily) for 14days. Platelet function was evaluated before and after therapy via light transmittance aggregometry and the VerifyNow P2Y12 assay. RESULTS: The adenosine diphosphate (ADP)-induced maximal extent of platelet aggregation differed significantly between the low-dose Ticagrelor and clopidogrel groups (ANCOVA, p=0.04 after stimulation with 5mumol/L ADP; p<0.01 after stimulation with 20mumol/L ADP). Inhibition of platelet aggregation increased significantly in the order of clopidogrel, low-dose Ticagrelor, and standard-dose Ticagrelor, as revealed by adjusted intergroup comparison analysis (ANCOVA, p=0.04 after stimulation with 5mumol/L ADP; p=0.005 after stimulation with 20mumol/L ADP). The rates of onset of the antiplatelet effect curves from 0 to 5h after administration of the LDs were greater in the standard- and low-dose Ticagrelor groups than in the clopidogrel group. Significant sequential reductions in P2Y12 reaction units were noted, in the following order: clopidogrel, low-dose Ticagrelor, and standard-dose Ticagrelor (ANCOVA, p<0.001). No bleeding occurred in the low-dose Ticagrelor group. CONCLUSIONS: Low-dose Ticagrelor afforded greater platelet inhibition than did clopidogrel in ESRD patients on HD.
Impact of Diabetes Mellitus on the Pharmacodynamic Effects of Ticagrelor Versus Clopidogrel in Troponin-Negative Acute Coronary Syndrome Patients Undergoing Ad Hoc Percutaneous Coronary Intervention.[Pubmed:28356282]
J Am Heart Assoc. 2017 Mar 29;6(4). pii: JAHA.117.005650.
BACKGROUND: Diabetes mellitus (DM) is associated with enhanced platelet reactivity and impaired response to oral antiplatelet therapy, including clopidogrel. This post hoc analysis investigated the pharmacodynamic effects of Ticagrelor versus clopidogrel loading dose (LD) in troponin-negative acute coronary syndrome patients with or without DM undergoing percutaneous coronary intervention in the Ad Hoc PCI study. METHODS AND RESULTS: Patients randomized (1:1) to receive Ticagrelor 180 mg LD or clopidogrel 600 mg LD were assessed by diabetic status. Platelet reactivity (P2Y12 reaction units [PRU] on VerifyNow((R)) assay) was measured pre-LD, at 0.5, 2, and 8 hours post-LD, and at the end of the percutaneous coronary intervention. The primary endpoint was PRU levels 2 hours post-LD; secondary endpoints included rates of high on-treatment platelet reactivity (PRU>/=208). Of 100 randomized patients, 51 received Ticagrelor (DM, n=20; non-DM, n=31) and 49 clopidogrel (DM, n=16; non-DM, n=33). At 2 hours post-LD, mean (SD) PRU levels in DM patients were 130.1 (111.7) with Ticagrelor versus 287.6 (71.9) with clopidogrel (mean [95%CI] difference -157.5 [-225.3, -89.8]; P<0.001); in non-DM patients, they were 75.3 (75.7) versus 243.0 (72.4) (mean difference -167.7 [-207.1, -128.3]; P<0.001). High on-treatment platelet reactivity rates at 2 hours post-LD were also significantly (P<0.001) reduced with Ticagrelor versus clopidogrel in DM and non-DM patients. Between-treatment differences for PRU and high on-treatment platelet reactivity were not significant at earlier time points but were at 8 hours post-LD (P<0.001). CONCLUSIONS: Compared with clopidogrel, Ticagrelor achieved faster, enhanced platelet inhibition and reduced high on-treatment platelet reactivity rates, in DM and non-DM patients. CLINICAL TRIAL REGISTRATION: URL: http://www.clinicaltrials.gov. Unique identifier: NCT01603082.


