Picroside ICAS# 27409-30-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 27409-30-9 | SDF | Download SDF |
| PubChem ID | 6440892 | Appearance | White powder |
| Formula | C24H28O11 | M.Wt | 492.47 |
| Type of Compound | Iridoids | Storage | Desiccate at -20°C |
| Synonyms | 6'-Cinnamoylcatalpol | ||
| Solubility | DMSO : 250 mg/mL (507.65 mM; Need ultrasonic) H2O : 125 mg/mL (253.82 mM; Need ultrasonic) | ||
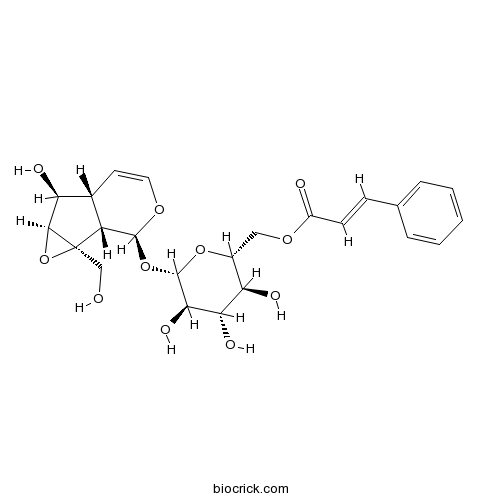
| Chemical Name | [(2R,3S,4S,5R,6S)-6-[[(1aS,1bS,2S,5aR,6S,6aS)-6-hydroxy-1a-(hydroxymethyl)-2,5a,6,6a-tetrahydro-1bH-oxireno[5,6]cyclopenta[1,3-c]pyran-2-yl]oxy]-3,4,5-trihydroxyoxan-2-yl]methyl (E)-3-phenylprop-2-enoate | ||
| SMILES | C1=CC=C(C=C1)C=CC(=O)OCC2C(C(C(C(O2)OC3C4C(C=CO3)C(C5C4(O5)CO)O)O)O)O | ||
| Standard InChIKey | XZGPUOQGERGURE-LUVHZPKESA-N | ||
| Standard InChI | InChI=1S/C24H28O11/c25-11-24-16-13(17(27)21(24)35-24)8-9-31-22(16)34-23-20(30)19(29)18(28)14(33-23)10-32-15(26)7-6-12-4-2-1-3-5-12/h1-9,13-14,16-23,25,27-30H,10-11H2/b7-6+/t13-,14-,16-,17+,18-,19+,20-,21+,22+,23+,24-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Picroside I is a hepatoprotective agent which is reported to be antimicrobial and used against hepatitis B. It has antioxidant, and anti-inflammatory activities, it may be the valuable anti-invasive drug candidates for cancer therapy by suppressing Collagenases and Gelatinases. Picroside I can enhance basic fibroblast growth factor(bFGF)-, staurosporine- or dbc-mitogen-activated protein (MAP)-induced neurite outgrowth from PC12D cells. |
| Targets | cAMP | MAPK | MMP(e.g.TIMP) |
| In vitro | Picrosides I and II, selective enhancers of the mitogen-activated protein kinase-dependent signaling pathway in the action of neuritogenic substances on PC12D cells.[Pubmed: 12151059]Life Sci. 2002 Aug 30;71(15):1821-35.
|
| In vivo | Antiinflammatory activity of the iridoids kutkin, picroside-1 and kutkoside from Picrorhiza kurrooa[Reference: WebLink]Phytother. Res., 1993, 7(6):402-7.Powdered roots of Picrorhiza kurrooa (PK), its alcoholic extract (AEPK) and active constituents kutkin, Picroside I and kutkoside demonstrated antiinflammatory activity (AIA) in a variety of test models. Significant AIA was recorded in adjuvant-induced and formaldehyde arthritis in rats and mice. In carrageenan-induced oedema inhibitory activity was remarkably enhanced upon intraperitoneal treatment in rats and mice. Kutkin exhibited significant action in dextran-induced oedema in rats. It inhibited acetic acid induced vascular permeability in mice and leucocyte migration in rats. Kutkin lacked any analgesic, antipyretic or ulcerogenic effect. |
| Kinase Assay | Picroliv, picroside-I and kutkoside from Picrorhiza kurrooa are scavengers of superoxide anions.[Pubmed: 1321626]Biochem Pharmacol. 1992 Jul 7;44(1):180-3.Picroliv, the active principle of Picrorhiza kurrooa, and its main components which are a mixture of the iridoid glycosides, Picroside I and kutkoside, were studied in vitro as potential scavengers of oxygen free radicals.
|
| Cell Research | Iridoid glycosides-Kutkin, Picroside I, and Kutkoside from Picrorrhiza kurroa Benth inhibits the invasion and migration of MCF-7 breast cancer cells through the down regulation of matrix metalloproteinases 1st Cancer Update[Reference: WebLink]Arab. J. Chem. 2011, 6(1):49-58.Aim of the study Here, MCF-7 cell lines (Human breast cancer) were used to test whether P. kurroa extract (PE) and its isolated iridoid glycosides Picroside I (PS), Kutkoside (KS), and Kutkin (KT) exerts the anti-invasion activity via down-regulation of the expression of matrix metalloproteinases (MMPs). MMPs play an important role in solid tumor invasion and migration.
|
| Structure Identification | Phytochem Anal. 2013 Nov-Dec;24(6):598-602.A proposed biosynthetic pathway of picrosides linked through the detection of biochemical intermediates in the endangered medicinal herb Picrorhiza kurroa.[Pubmed: 23696248]Picrorhiza kurroa Royle ex Benth is an important medicinal herb used in the preparation of several herbal drug formulations due to the presence of Picroside I (P-I) and picroside-II (P-II) along with other iridoid-glucosides derivatives.
The endangered status of P. kurroa coupled with lack of information on biosynthesis of Picroside Iand P-II necessitate deciphering the biosynthetic pathway for picrosides. |

Picroside I Dilution Calculator

Picroside I Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0306 mL | 10.1529 mL | 20.3058 mL | 40.6116 mL | 50.7645 mL |
| 5 mM | 0.4061 mL | 2.0306 mL | 4.0612 mL | 8.1223 mL | 10.1529 mL |
| 10 mM | 0.2031 mL | 1.0153 mL | 2.0306 mL | 4.0612 mL | 5.0765 mL |
| 50 mM | 0.0406 mL | 0.2031 mL | 0.4061 mL | 0.8122 mL | 1.0153 mL |
| 100 mM | 0.0203 mL | 0.1015 mL | 0.2031 mL | 0.4061 mL | 0.5076 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- threo-Guaiacylglycerol
Catalog No.:BCN5161
CAS No.:27391-16-8
- 4'-Hydroxy-7-methoxyflavan
Catalog No.:BCN3497
CAS No.:27348-54-5
- VX-765
Catalog No.:BCC3648
CAS No.:273404-37-8
- Precyasterone
Catalog No.:BCN2754
CAS No.:27335-85-9
- 5-Formyl-2-furylboronic acid
Catalog No.:BCC8748
CAS No.:27329-70-0
- Tirapazamine
Catalog No.:BCC5184
CAS No.:27314-97-2
- ICI 63197
Catalog No.:BCC7188
CAS No.:27277-00-5
- Phyllostine
Catalog No.:BCN4773
CAS No.:27270-89-9
- Levobupivacaine HCl
Catalog No.:BCC4675
CAS No.:27262-48-2
- N-(2,6-Dimethylphenyl)-2-piperidinecarboxamide
Catalog No.:BCC9051
CAS No.:27262-40-4
- Neoandrographolide
Catalog No.:BCN4657
CAS No.:27215-14-1
- Cyanidin 3-Arabinoside
Catalog No.:BCC8157
CAS No.:27214-72-8
- Coumarin 7
Catalog No.:BCC8920
CAS No.:27425-55-4
- 5-Methoxyisatin
Catalog No.:BCC8098
CAS No.:39755-95-8
- Xanthohumol D
Catalog No.:BCN5162
CAS No.:274675-25-1
- Neocryptotanshinone II
Catalog No.:BCN3138
CAS No.:27468-20-8
- Ticagrelor
Catalog No.:BCC4975
CAS No.:274693-27-5
- LE 300
Catalog No.:BCC7148
CAS No.:274694-98-3
- 7-Acetoxy-4-methylcoumarin
Catalog No.:BCC8775
CAS No.:2747-05-9
- H-Leu-OtBu.HCl
Catalog No.:BCC2974
CAS No.:2748-02-9
- O-Benzyldauricine
Catalog No.:BCC8222
CAS No.:2748-99-4
- Cyclo(D-Val-L-Pro)
Catalog No.:BCN4015
CAS No.:27483-18-7
- trans-4-Aminocyclohexanol
Catalog No.:BCC9181
CAS No.:27489-62-9
- L-Ala-ol
Catalog No.:BCC2590
CAS No.:2749-11-3
Picrosides I and II, selective enhancers of the mitogen-activated protein kinase-dependent signaling pathway in the action of neuritogenic substances on PC12D cells.[Pubmed:12151059]
Life Sci. 2002 Aug 30;71(15):1821-35.
Picrosides I and II caused a concentration-dependent (> 0.1 microM) enhancement of basic fibroblast growth factor (bFGF, 2 ng/ml)-, staurosporine (10 nM)- and dibutyryl cyclic AMP (dbcAMP, 0.3 mM)-induced neurite outgrowth from PC12D cells. PD98059 (20 microM), a potent mitogen-activated protein (MAP) kinase kinase inhibitor, blocked the enhancement of bFGF (2 ng/ml)-, staurosporine (10 nM)- or dbcAMP (0.3 mM)-induced neurite outgrowth by picrosides, suggesting that picrosides activate MAP kinase-dependent signaling pathway. However, PD98059 did not affect the bFGF (2 ng/ml)-, staurosporine (10 nM)- and dbcAMP (0.3 mM)-induced neurite outgrowth in PC12D cells, indicating the existence of two components in neurite outgrowth induced by bFGF, staurosporine and dbcAMP, namely the MAP kinase-independent and the masked MAP kinase-dependent one. Furthermore, picrosides-induced enhancements of the bFGF-action were markedly inhibited by GF109203X (0.1 microM), a protein kinase C inhibitor. The expression of phosphorylated MAP kinase was markedly increased by bFGF (2 ng/ml) and dbcAMP (0.3 mM), whereas that was not enhanced by staurosporine (10 nM). Picrosides had no effect on the phosphorylation of MAP kinase induced by bFGF or dbcAMP and also unaffected it in the presence of staurosporine. These results suggest that picrosides I and II enhance bFGF-, staurosporine- or dbcAMP-induced neurite outgrowth from PC12D cells, probably by amplifying a down-stream step of MAP kinase in the intracellular MAP kinase-dependent signaling pathway. Picrosides I and II may become selective pharmacological tools for studying the MAP kinase-dependent signaling pathway in outgrowth of neurites induced by many kinds of neuritogenic substances including bFGF.
Picroliv, picroside-I and kutkoside from Picrorhiza kurrooa are scavengers of superoxide anions.[Pubmed:1321626]
Biochem Pharmacol. 1992 Jul 7;44(1):180-3.
Picroliv, the active principle of Picrorhiza kurrooa, and its main components which are a mixture of the iridoid glycosides, picroside-I and kutkoside, were studied in vitro as potential scavengers of oxygen free radicals. The superoxide (O2-) anions generated in a xanthine-xanthine oxidase system, as measured in terms of uric acid formed and the reduction of nitroblue tetrazolium were shown to be suppressed by picroliv, picroside-I and kutkoside. Picroliv as well as both glycosides inhibited the non-enzymic generation of O2- anions in a phenazine methosulphate NADH system. Malonaldehyde (MDA) generation in rat liver microsomes as stimulated by both the ascorbate-Fe2+ and NADPH-ADP-Fe2+ systems was shown to be inhibited by the Picroliv glycosides. Known antioxidants tocopherol (vitamin E) and butylated hydroxyanisole (BHA) were also compared with regard to their antioxidant actions in the above system. It was found that BHA afforded protection against ascorbate-Fe(2+)-induced MDA formation in microsomes but did not interfere with enzymic or non-enzymic O2- anion generation; and tocopherol inhibited lipid peroxidation in microsomes by both prooxidant systems and the generation of O2- anions in the non-enzymic system but did not interfere with xanthine oxidase activity. The present study shows that picroliv, picroside-I and kutkoside possess the properties of antioxidants which appear to be mediated through activity like that of superoxide dismutase, metal ion chelators and xanthine oxidase inhibitors.
A proposed biosynthetic pathway of picrosides linked through the detection of biochemical intermediates in the endangered medicinal herb Picrorhiza kurroa.[Pubmed:23696248]
Phytochem Anal. 2013 Nov-Dec;24(6):598-602.
INTRODUCTION: Picrorhiza kurroa Royle ex Benth is an important medicinal herb used in the preparation of several herbal drug formulations due to the presence of picroside-I (P-I) and picroside-II (P-II) along with other iridoid-glucosides derivatives. OBJECTIVE: The endangered status of P. kurroa coupled with lack of information on biosynthesis of P-I and P-II necessitate deciphering the biosynthetic pathway for picrosides. METHODS: LC with electrospray ionisation (ESI) and quadrupole time of flight combined with MS/MS was used to detect intermediates and assemble the picrosides biosynthetic pathway in P. kurroa. RESULTS: The presence of catalpol and aucubin, the major backbone structures of picrosides, along with intermediate metabolites boschnaloside, bartsioside and mussaenosidic acid, was confirmed in ESI negative mode with pseudomolecular ion peaks, that is, m/z 361, m/z 343, m/z 345, m/z 329 and m/z 375 ions and their fragmentation patterns. CONCLUSION: The picrosides biosynthetic pathway is expected to provide a reliable platform towards understanding the molecular components (genes/enzymes) of P-I and P-II biosynthesis in P. kurroa for their eventual utilisation in various applications.


