TectolCAS# 24449-39-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 24449-39-6 | SDF | Download SDF |
| PubChem ID | 161453 | Appearance | Powder |
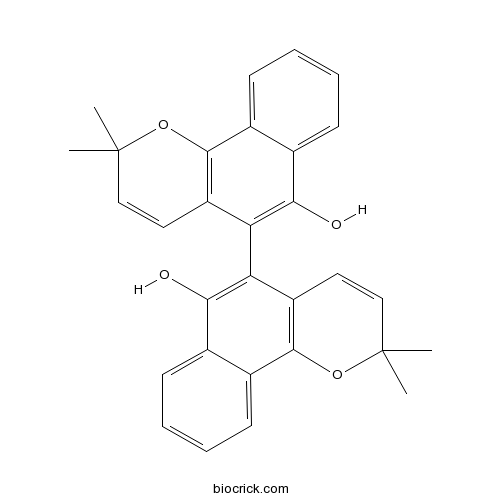
| Formula | C30H26O4 | M.Wt | 450.5 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-(6-hydroxy-2,2-dimethylbenzo[h]chromen-5-yl)-2,2-dimethylbenzo[h]chromen-6-ol | ||
| SMILES | CC1(C=CC2=C(O1)C3=CC=CC=C3C(=C2C4=C(C5=CC=CC=C5C6=C4C=CC(O6)(C)C)O)O)C | ||
| Standard InChIKey | FVTJXDIACKJEPH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C30H26O4/c1-29(2)15-13-21-23(25(31)17-9-5-7-11-19(17)27(21)33-29)24-22-14-16-30(3,4)34-28(22)20-12-8-6-10-18(20)26(24)32/h5-16,31-32H,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tectol has anti-plasmodial activity, it as a moderately active growth inhibitor with an IC50 3.44±0.20μM. It exhibited significant activity against human leukemia cell lines HL60 and CEM. Tectol may cause itchy dermatitis, the dermatitis appeared on the neck, upper legs, underarms, and trunk area. |
| In vitro | Chemical constituents from Lippia sidoides and cytotoxic activity.[Pubmed: 11421746 ]J Nat Prod. 2001 Jun;64(6):792-5.
|
| In vivo | Allergy to spermicidal lubricant in a contraceptive.[Pubmed: 3652700 ]Contact Dermatitis. 1987 Aug;17(2):115-6.
|
| Structure Identification | Bioorg Med Chem. 2016 Jul 15;24(14):3102-7.Discovery and preliminary structure-activity relationship studies on tecomaquinone I and tectol as novel farnesyltransferase and plasmodial inhibitors.[Pubmed: 27240468 ]
Biological screening of a library of synthesized benzo[c]chromene-7,10-dione natural products against human farnesyltransferase (FTase) has identified tecomaquinone I (IC50 of 0.065±0.004μM) as being one of the more potent natural product inhibitors identified to date.
|

Tectol Dilution Calculator

Tectol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2198 mL | 11.0988 mL | 22.1976 mL | 44.3951 mL | 55.4939 mL |
| 5 mM | 0.444 mL | 2.2198 mL | 4.4395 mL | 8.879 mL | 11.0988 mL |
| 10 mM | 0.222 mL | 1.1099 mL | 2.2198 mL | 4.4395 mL | 5.5494 mL |
| 50 mM | 0.0444 mL | 0.222 mL | 0.444 mL | 0.8879 mL | 1.1099 mL |
| 100 mM | 0.0222 mL | 0.111 mL | 0.222 mL | 0.444 mL | 0.5549 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Perisesaccharide B
Catalog No.:BCN8573
CAS No.:1095261-93-0
- 4'-O-Methylochnaflavone
Catalog No.:BCN8572
CAS No.:49619-87-6
- 2-Amino-3-carboxy-1,4-naphthoquinone
Catalog No.:BCN8571
CAS No.:173043-38-4
- Graveobioside A
Catalog No.:BCN8570
CAS No.:506410-53-3
- Gancaonin N
Catalog No.:BCN8569
CAS No.:129145-52-4
- Saikogenin D
Catalog No.:BCN8568
CAS No.:5573-16-0
- Vinaginsenoside R8
Catalog No.:BCN8567
CAS No.:156042-22-7
- 3-Feruloyl-1-Sinapoyl sucrose
Catalog No.:BCN8566
CAS No.:98942-06-4
- Periplocoside N
Catalog No.:BCN8565
CAS No.:39946-41-3
- Lancifodilactone F
Catalog No.:BCN8564
CAS No.:850878-47-6
- 3'-Demethylnobiletin
Catalog No.:BCN8563
CAS No.:112448-39-2
- Erigoster B
Catalog No.:BCN8562
CAS No.:849777-61-3
- Jionoside D
Catalog No.:BCN8575
CAS No.:120406-34-0
- 5,6-Dehydroginsenoside Rd
Catalog No.:BCN8576
CAS No.:1268459-68-2
- Epitheaflagallin 3-O-gallate
Catalog No.:BCN8577
CAS No.:102067-92-5
- Hexamethylquercetagetin
Catalog No.:BCN8578
CAS No.:1251-84-9
- 4-Benzamido-2,5-diethoxybenzenediazonium
Catalog No.:BCN8579
CAS No.:5486-84-0
- Uvarigrin
Catalog No.:BCN8580
CAS No.:200563-11-7
- Isoginsenoside Rh3
Catalog No.:BCN8581
CAS No.:166040-90-0
- Isorosmanol
Catalog No.:BCN8582
CAS No.:93780-80-4
- Ligupurpuroside C
Catalog No.:BCN8583
CAS No.:1194056-33-1
- Lucidin 3-O-primeveroside
Catalog No.:BCN8584
CAS No.:29706-59-0
- Platycodin D3
Catalog No.:BCN8585
CAS No.:67884-03-1
- Sarracenin
Catalog No.:BCN8586
CAS No.:59653-37-1
Discovery and preliminary structure-activity relationship studies on tecomaquinone I and tectol as novel farnesyltransferase and plasmodial inhibitors.[Pubmed:27240468]
Bioorg Med Chem. 2016 Jul 15;24(14):3102-7.
Biological screening of a library of synthesized benzo[c]chromene-7,10-dione natural products against human farnesyltransferase (FTase) has identified tecomaquinone I (IC50 of 0.065+/-0.004muM) as being one of the more potent natural product inhibitors identified to date. Anti-plasmodial screening of the same library against a drug-resistant strain of Plasmodium falciparum identified the structurally-related dichromenol Tectol as a moderately active growth inhibitor with an IC50 3.44+/-0.20muM. Two novel series of analogues, based on the benzo[c]chromene-7,10-dione scaffold, were subsequently synthesized, with one analogue exhibiting farnesyltransferase inhibitory activity in the low micromolar range. A preliminary structure-activity relationship (SAR) study has identified different structural requirements for anti-malarial activity in comparison to FTase activities for these classes of natural products. Our results identify tecomaquinone I as a novel scaffold from which more potent inhibitors of human and parasitic FTase could be developed.
Chemical constituents from Lippia sidoides and cytotoxic activity.[Pubmed:11421746]
J Nat Prod. 2001 Jun;64(6):792-5.
Eleven known compounds and a new prenylated naphthoquinone, lippsidoquinone (13), were isolated from ethanol extracts of Lippia sidoides. Their structures were established by a combination of 1D and 2D NMR, IR, and EIMS spectral data analysis. The cytotoxic properties of compounds 3--13 were evaluated against HL60, SW1573, and CEM cell lines. Only Tectol (6) and lippsidoquinone (13) exhibited significant activity against human leukemia cell lines HL60 and CEM.


