Taccalonolide ACAS# 108885-68-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 108885-68-3 | SDF | Download SDF |
| PubChem ID | 441685 | Appearance | Powder |
| Formula | C36H46O14 | M.Wt | 702.74 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
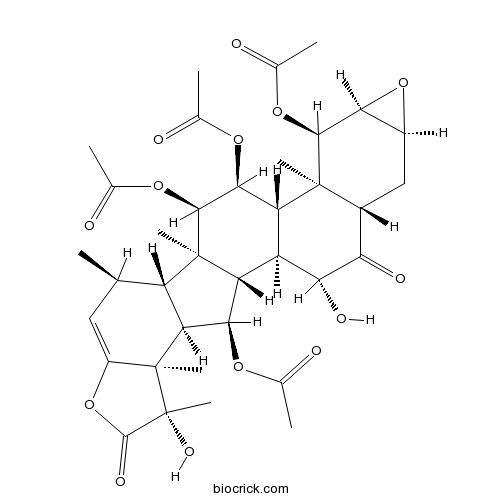
| SMILES | CC1C=C2C(C3C1C4(C(C3OC(=O)C)C5C(C(C4OC(=O)C)OC(=O)C)C6(C(CC7C(C6OC(=O)C)O7)C(=O)C5O)C)C)(C(C(=O)O2)(C)O)C | ||
| Standard InChIKey | PTTJLTMUKRRHAT-VJAKQJMOSA-N | ||
| Standard InChI | InChI=1S/C36H46O14/c1-12-10-19-35(8,36(9,44)32(43)50-19)24-21(12)34(7)22(28(24)45-13(2)37)20-23(29(46-14(3)38)31(34)48-16(5)40)33(6)17(25(41)26(20)42)11-18-27(49-18)30(33)47-15(4)39/h10,12,17-18,20-24,26-31,42,44H,11H2,1-9H3/t12-,17-,18+,20+,21+,22-,23-,24+,26-,27+,28-,29+,30+,31+,33+,34-,35+,36-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Taccalonolide A is the first microtubule stabilizing agent to be discovered from a plant since identification of the mechanism of action of paclitaxel and it is the first natural product steroid identified to have these cellular effects. |
| Targets | Bcl-2/Bax | Caspase | P-gp |
| Kinase Assay | Taccalonolide microtubule stabilizing agents[Reference: WebLink]April 12, 2005In accordance with the present invention, there have been identified extracts from a tropical plant that initiate paclitaxel-like microtubule bundling. |

Taccalonolide A Dilution Calculator

Taccalonolide A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.423 mL | 7.115 mL | 14.23 mL | 28.46 mL | 35.575 mL |
| 5 mM | 0.2846 mL | 1.423 mL | 2.846 mL | 5.692 mL | 7.115 mL |
| 10 mM | 0.1423 mL | 0.7115 mL | 1.423 mL | 2.846 mL | 3.5575 mL |
| 50 mM | 0.0285 mL | 0.1423 mL | 0.2846 mL | 0.5692 mL | 0.7115 mL |
| 100 mM | 0.0142 mL | 0.0712 mL | 0.1423 mL | 0.2846 mL | 0.3558 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lupeol 3-hydroxyoctadecanoate
Catalog No.:BCN6686
CAS No.:108885-61-6
- Gardenolic acid B
Catalog No.:BCN7140
CAS No.:108864-53-5
- Dendrophenol
Catalog No.:BCC8165
CAS No.:108853-14-1
- Nemorubicin
Catalog No.:BCC4151
CAS No.:108852-90-0
- Isomeranzin
Catalog No.:BCN5882
CAS No.:1088-17-1
- 1-O-Acetyl-6beta-O-Isobutyrylbritannilactone
Catalog No.:BCN1628
CAS No.:1087072-50-1
- Y 11
Catalog No.:BCC6206
CAS No.:1086639-59-9
- Eupalinolide K
Catalog No.:BCN6199
CAS No.:108657-10-9
- Purpureaside C
Catalog No.:BCN3865
CAS No.:108648-07-3
- Mizolastine
Catalog No.:BCC4521
CAS No.:108612-45-9
- GSK2126458
Catalog No.:BCC3884
CAS No.:1086062-66-9
-
4-Hydroxy-Teriflunomide
Catalog No.:BCC4734
CAS No.:
- Taccalonolide B
Catalog No.:BCN2743
CAS No.:108885-69-4
- 13-O-Acetylcorianin
Catalog No.:BCN5883
CAS No.:108887-44-1
- GSK-923295
Catalog No.:BCC1608
CAS No.:1088965-37-0
- MK 6096
Catalog No.:BCC4020
CAS No.:1088991-73-4
- Soyasaponin IV
Catalog No.:BCN1627
CAS No.:108906-97-4
- U0124
Catalog No.:BCC7200
CAS No.:108923-79-1
- GSK1904529A
Catalog No.:BCC1062
CAS No.:1089283-49-7
- H-7 dihydrochloride
Catalog No.:BCC6686
CAS No.:108930-17-2
- Neocurdione
Catalog No.:BCC9242
CAS No.:108944-67-8
- FURA-2AM
Catalog No.:BCC7296
CAS No.:108964-32-5
- N-Valeric acid
Catalog No.:BCC8220
CAS No.:109-52-4
- Allylthiourea
Catalog No.:BCC4759
CAS No.:109-57-9
Antitrypanosomal Activity of a Novel Taccalonolide from the Tubers of Tacca leontopetaloides.[Pubmed:27313159]
Phytochem Anal. 2016 May;27(3-4):217-21.
INTRODUCTION: Several taccalonolides with various bioactivities have been isolated from Tacca species but no studies to isolate taccalonolides with anti-trypanosomal activity from Tacca leontopetaloides have been reported. OBJECTIVES: To analyse extracts of the roots of Tacca leontopetaloides, purify the extracts by column chromatography and identify isolated compounds by spectroscopic methods. The compounds and fractions will be tested for antitrypanosomal activity in vitro against Trypanosoma brucei brucei. MATERIAL AND METHODS: Dried roots or tubers of Tacca leontopetaloides, chromatographic separation and spectroscopic identification. RESULTS: A novel Taccalonolide A propanoate and some known taccalonolides were isolated and their structures were determined by NMR and mass spectrometry CONCLUSION: Several taccalonolides were isolated from Tacca leontopetaloides and were found to have in vitro antitrypanosomal activity against Trypanosoma brucei brucei and EC50 values for the isolated compounds were from 0.79 microg/mL. Copyright (c) 2016 John Wiley & Sons, Ltd.
Synthetic reactions with rare taccalonolides reveal the value of C-22,23 epoxidation for microtubule stabilizing potency.[Pubmed:24959756]
J Med Chem. 2014 Jul 24;57(14):6141-9.
The taccalonolides are microtubule stabilizers isolated from plants of the genus Tacca. Taccalonolide AF is 231 times more potent than the major metabolite Taccalonolide A and differs only by the oxidation of the C-22,23 double bond in A to an epoxy group in AF. In the current study, 10 other rare natural taccalonolides were epoxidized and in each case epoxidation improved potency. The epoxidation products of taccalonolide T and AI were the most potent, with IC50 values of 0.43 and 0.88 nM, respectively. These potent taccalonolides retained microtubule stabilizing effects, and T-epoxide demonstrated antitumor effects in a xenograft model of breast cancer. Additional reactions demonstrated that reduction of the C-6 ketone resulted in an inactive Taccalonolide And that C-22,23 epoxidation restored its activity. These studies confirm the value of C-22,23 epoxidation as an effective strategy for increasing the potency of a wide range of structurally diverse taccalonolide microtubule stabilizers.
Taccalonolide microtubule stabilizers.[Pubmed:24491636]
Bioorg Med Chem. 2014 Sep 15;22(18):5091-6.
This review focuses on a relatively new class of microtubule stabilizers, the taccalonolides. The taccalonolides are highly oxygenated pentacyclic steroids isolated from plants of the genus Tacca. Originally identified in a cell-based phenotypic screen, the taccalonolides have many properties similar to other microtubule stabilizers. They increase the density of interphase microtubules, causing microtubule bundling, and form abnormal multi-polar mitotic spindles leading to mitotic arrest and, ultimately, apoptosis. However, the taccalonolides differ from other microtubule stabilizers in that they retain efficacy in taxane resistant cell lines and in vivo models. Binding studies with the newly identified, potent Taccalonolide AJ demonstrated covalent binding to beta-tubulin at or near the luminal and/or pore taxane binding site(s) which stabilizes microtubule protofilaments in a unique manner as compared to other microtubule stabilizers. The isolation and semi-synthesis of 21 taccalonolides helped to identify key structure activity relationships and the importance of multiple regions across the taccalonolide skeleton for optimal biological potency.
Hydrolysis reactions of the taccalonolides reveal structure-activity relationships.[Pubmed:23855953]
J Nat Prod. 2013 Jul 26;76(7):1369-75.
The taccalonolides are microtubule stabilizers isolated from plants of the genus Tacca that show potent in vivo antitumor activity and the ability to overcome multiple mechanisms of drug resistance. The most potent taccalonolide identified to date, AJ, is a semisynthetic product generated from the major plant metabolite Taccalonolide A in a two-step reaction. The first step involves hydrolysis of Taccalonolide A to generate taccalonolide B, and then this product is oxidized to generate an epoxide group at C-22-C-23. To generate sufficient Taccalonolide AJ for in vivo antitumor efficacy studies, the hydrolysis conditions for the conversion of Taccalonolide A to B were optimized. During purification of the hydrolysis products, we identified the new Taccalonolide AO (1) along with taccalonolide I. When the same hydrolysis reaction was performed on a taccalonolide E-enriched fraction, four new taccalonolides, assigned as AK, AL, AM, and AN (2-5), were obtained in addition to the expected product taccalonolide N. Biological assays were performed on each of the purified taccalonolides, which allowed for increased refinement of the structure-activity relationship of this class of compounds.
Identification and biological activities of new taccalonolide microtubule stabilizers.[Pubmed:21800839]
J Med Chem. 2011 Sep 8;54(17):6117-24.
The taccalonolides are a unique class of microtubule stabilizers that do not bind directly to tubulin. Three new taccalonolides, Z, AA, and AB, along with two known compounds, taccalonolides R and T, were isolated from Tacca chantrieri and Tacca integrifolia. Taccalonolide structures were determined by 1D and 2D NMR methods. The biological activities of the new taccalonolides, as well as taccalonolides A, B, E, N, R, and T, were evaluated. All nine taccalonolides display microtubule stabilizing activity, but profound differences in antiproliferative potencies were noted, with IC(50) values ranging from the low nanomolar range for Taccalonolide AA (32 nM) to the low micromolar range for taccalonolide R (13 muM). These studies demonstrate that diverse taccalonolides possess microtubule stabilizing properties and that significant structure-activity relationships exist. In vivo antitumor evaluations of taccalonolides A, E, and N show that each of these molecules has in vivo antitumor activity.
Cellular studies reveal mechanistic differences between taccalonolide A and paclitaxel.[Pubmed:21597323]
Cell Cycle. 2011 Jul 1;10(13):2162-71.
Taccalonolide A is a microtubule stabilizer that has cellular effects almost identical to paclitaxel. However, biochemical studies show that, unlike paclitaxel, Taccalonolide A does not enhance purified tubulin polymerization or bind tubulin/microtubules. Mechanistic studies aimed at understanding the nature of the differences between Taccalonolide A and paclitaxel were conducted. Our results show that Taccalonolide A causes bundling of interphase microtubules at concentrations that cause antiproliferative effects. In contrast, the concentration of paclitaxel that initiates microtubule bundling is 31-fold higher than its IC 50. Taccalonolide A's effects are further differentiated from paclitaxel in that it is unable to enhance the polymerization of tubulin in cellular extracts. This finding extends previous biochemical results with purified brain tubulin to demonstrate that Taccalonolide A requires more than tubulin and a full complement of cytosolic proteins to cause microtubule stabilization. Reversibility studies were conducted and show that the cellular effects of Taccalonolide A persist after drug washout. In contrast, other microtubule stabilizers, including paclitaxel and laulimalide, demonstrate a much higher degree of cellular reversibility in both short-term proliferation and long-term clonogenic assays. The propensity of Taccalonolide A to alter interphase microtubules at antiproliferative concentrations as well as its high degree of cellular persistence may explain why Taccalonolide A is more potent in vivo than would be expected from cellular studies. The close linkage between the microtubule bundling and antiproliferative effects of Taccalonolide A is of interest given the recent hypothesis that the effects of microtubule targeting agents on interphase microtubules might play a prominent role in their clinical anticancer efficacy.
The taccalonolides, novel microtubule stabilizers, and gamma-radiation have additive effects on cellular viability.[Pubmed:21507571]
Cancer Lett. 2011 Aug 1;307(1):104-111.
The taccalonolides are novel antimitotic microtubule stabilizers that have a unique mechanism of action independent of a direct interaction with tubulin. Cytotoxicity and clonogenic assays show that Taccalonolide A and radiation act in an additive manner to cause cell death. The taxanes and epothilones have utility when combined with radiotherapy and these findings further suggest the additive effects of microtubule targeting agents with radiation on cellular proliferation are independent of direct tubulin binding and are instead a result of the downstream effects of these agents. These studies suggest that diverse antimitotic agents, including the taccalonolides, may have utility in chemoradiotherapy.
Taccalonolides E and A: Plant-derived steroids with microtubule-stabilizing activity.[Pubmed:12810650]
Cancer Res. 2003 Jun 15;63(12):3211-20.
During the course of a mechanism-based screening program designed to identify new microtubule-disrupting agents from natural products, we identified a crude extract from Tacca chantrieri that initiated Taxol-like microtubule bundling. Bioassay-directed purification of the extract yielded the highly oxygenated steroids taccalonolides E and A. The taccalonolides caused an increased density of cellular microtubules in interphase cells and the formation of thick bundles of microtubules similar to the effects of Taxol. Mitotic cells exhibited abnormal mitotic spindles containing three or more spindle poles. The taccalonolides were evaluated for antiproliferative effects in drug-sensitive and multidrug-resistant cell lines. The data indicate that taccalonolide E is slightly more potent than Taccalonolide A in drug-sensitive cell lines and that both taccalonolides are effective inhibitors of cell proliferation. Both taccalonolides are poorer substrates for transport by P-glycoprotein than Taxol. The ability of the taccalonolides to circumvent mutations in the Taxol-binding region of beta-tubulin was examined using the PTX 10, PTX 22, and 1A9/A8 cell lines. The data suggest little cross-resistance of Taccalonolide A as compared with Taxol, however, the data from the PTX 22 cell line indicate a 12-fold resistance to taccalonolide E, suggesting a potential overlap of binding sites. Characteristic of agents that disrupt microtubules, the taccalonolides caused G(2)-M accumulation, Bcl-2 phosphorylation, and initiation of apoptosis. The taccalonolides represent a novel class of plant-derived microtubule-stabilizers that differ structurally and biologically from other classes of microtubule-stabilizers.


