TC-DAPK 6DAPK inhibitor CAS# 315694-89-4 |
- Nadifloxacin
Catalog No.:BCC4804
CAS No.:124858-35-1
- Calcipotriol monohydrate
Catalog No.:BCC1445
CAS No.:147657-22-5
- Halobetasol Propionate
Catalog No.:BCC4664
CAS No.:66852-54-8
- Dihydroartemisinin
Catalog No.:BCN6264
CAS No.:71939-50-9
Quality Control & MSDS
Number of papers citing our products

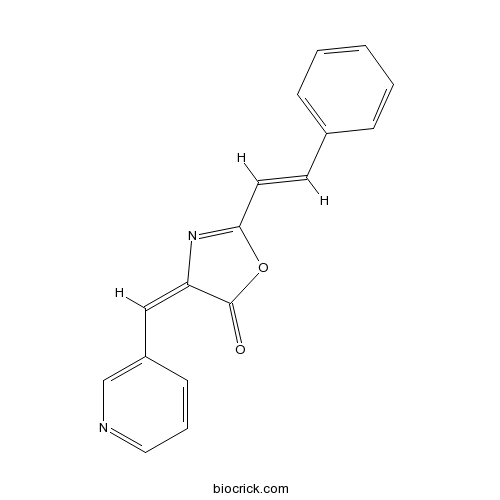
Chemical structure
3D structure
| Cas No. | 315694-89-4 | SDF | Download SDF |
| PubChem ID | 5676111 | Appearance | Powder |
| Formula | C17H12N2O2 | M.Wt | 276.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | DAPK inhibitor | ||
| Solubility | DMSO : 18.67 mg/mL (67.57 mM; Need ultrasonic and warming) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | (4E)-2-[(E)-2-phenylethenyl]-4-(pyridin-3-ylmethylidene)-1,3-oxazol-5-one | ||
| SMILES | C1=CC=C(C=C1)C=CC2=NC(=CC3=CN=CC=C3)C(=O)O2 | ||
| Standard InChIKey | GFGMISOSPOPSHN-QCTDOKRBSA-N | ||
| Standard InChI | InChI=1S/C17H12N2O2/c20-17-15(11-14-7-4-10-18-12-14)19-16(21-17)9-8-13-5-2-1-3-6-13/h1-12H/b9-8+,15-11+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | TC-DAPK 6 is a potent, ATP-competitive, and highly selective DAPK inhibitor (IC50=69 and 225 nM against DAPK1 and DAPK3, respectively, with 10 μM ATP).In Vitro:TC-DAPK 6 is found to be the most potent Death-associated protein kinase (DAPK) inhibitor with enzyme selectivity. When assayed with 10 μM ATP, the IC50 values for DAPK1 and DAPK3 are 69 and 225 nM, respectively. TC-DAPK 6 also inhibits p70S6K (1 μM < IC50< 10 μM)[1]. References: | |||||

TC-DAPK 6 Dilution Calculator

TC-DAPK 6 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6194 mL | 18.0969 mL | 36.1939 mL | 72.3877 mL | 90.4846 mL |
| 5 mM | 0.7239 mL | 3.6194 mL | 7.2388 mL | 14.4775 mL | 18.0969 mL |
| 10 mM | 0.3619 mL | 1.8097 mL | 3.6194 mL | 7.2388 mL | 9.0485 mL |
| 50 mM | 0.0724 mL | 0.3619 mL | 0.7239 mL | 1.4478 mL | 1.8097 mL |
| 100 mM | 0.0362 mL | 0.181 mL | 0.3619 mL | 0.7239 mL | 0.9048 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Death-associated protein kinase (DAPK) is a serine/threonine protein kinase implicated in diverse programmed cell death pathways. DAPK is a promising target protein for the treatment of ischemic diseases. TC-DAPK 6 is an oxazalone compound that acts as a potent, ATP-competitive, and highly selective death-associated protein kinase (DAPK) inhibitor (IC50 = 69 and 225 nM against DAPK1 and DAPK3, respectively, with 10 μM ATP); while exhibiting much reduced or no activity against a panel of 48 other kinases even at concentrations as high as 10 μM.
- Kavain
Catalog No.:BCN8295
CAS No.:3155-48-4
- 1,18-Octadecanediol
Catalog No.:BCN5233
CAS No.:3155-43-9
- Matsukaze-lactone
Catalog No.:BCN7580
CAS No.:3153-73-9
- O-Nornuciferine
Catalog No.:BCN7074
CAS No.:3153-55-7
- 5-Hydroxyseselin
Catalog No.:BCN3428
CAS No.:31525-75-4
- Isobavachin
Catalog No.:BCN5232
CAS No.:31524-62-6
- Sutherlandin trans-p-coumarate
Catalog No.:BCN5231
CAS No.:315236-68-1
- Ifflaiamine
Catalog No.:BCN7061
CAS No.:31520-95-3
- PAC-1
Catalog No.:BCC3600
CAS No.:315183-21-2
- Acetylheliosupine
Catalog No.:BCN1981
CAS No.:31514-30-4
- Testosterone enanthate
Catalog No.:BCC9169
CAS No.:315-37-7
- Allopurinol
Catalog No.:BCC3720
CAS No.:315-30-0
- STF-62247
Catalog No.:BCC4960
CAS No.:315702-99-9
- JK 184
Catalog No.:BCC3936
CAS No.:315703-52-7
- PTC-209
Catalog No.:BCC5111
CAS No.:315704-66-6
- 4EGI-1
Catalog No.:BCC5337
CAS No.:315706-13-9
- Heraclenol
Catalog No.:BCN5234
CAS No.:31575-93-6
- Berberine Sulphate
Catalog No.:BCC8131
CAS No.:316-41-6
- Emetine dihydrochloride
Catalog No.:BCN8307
CAS No.:316-42-7
- Z-Asp(OMe)-OH
Catalog No.:BCC2790
CAS No.:3160-47-2
- Z-Ile-OH
Catalog No.:BCC2593
CAS No.:3160-59-6
- Moroxydine HCl
Catalog No.:BCC4802
CAS No.:3160-91-6
- SCH 442416
Catalog No.:BCC7372
CAS No.:316173-57-6
- Artocarpesin
Catalog No.:BCN8071
CAS No.:3162-09-2
Role of vitamin D in energy and bone metabolism in postmenopausal women with type 2 diabetes mellitus: A 6-month follow-up evaluation.[Pubmed:28371517]
J Diabetes Investig. 2018 Jan;9(1):211-222.
AIMS/INTRODUCTION: Resting energy expenditure was associated with a serum bone turnover marker in postmenopausal women with type 2 diabetes (T2DMPW) in the present cross-sectional study. To clarify the fundamental pathological factor for the correlation of bone metabolism and basal metabolism in type 2 diabetes, a 6-month prospective follow-up study was carried out with supplementation of vitamin D. MATERIALS AND METHODS: A total of 44 T2DMPW were enrolled. The following factors were evaluated at the beginning and the end of the summer: procollagen type 1 N-terminal propeptide, carboxy-terminal collagen crosslinks-1, intact parathyroid hormone and 25-hydroxyvitamin D (25[OH]D), as well as diabetic complications, body composition, respiratory quotient and resting energy expenditure. A total of 23 patients with low 25(OH)D levels (<20 ng/mL) were instructed to increase vitamin D levels by lifestyle change. Among them, 15 patients with osteoporosis were also administered alfacalcidol. RESULTS: Serum 25(OH)D increased in 25 patients and decreased in 19 patients. Patients who did not receive the study intervention at the start tended to have a decreased 2525(OH)D level; therefore, the average 25(OH)D level of all patients was not changed. Changes in resting energy expenditure were positively correlated with those of procollagen type 1 N-terminal propeptide/carboxy-terminal collagen crosslinks-1. Changes in the respiratory quotient correlated with the mean glycated hemoglobin levels; procollagen type 1 N-terminal propeptide levels positively correlated with serum 25(OH)D after the intervention. These correlations were prominent in patients with increased 25(OH)D and those with alfacalcidol supplementation. CONCLUSIONS: Restoration of vitamin D level might be a prerequisite for a normal correlation between bone and basal metabolism in T2DMPW. Lifestyle intervention for retention of vitamin D level is important even in summer, in T2DMPW.
Binding S0.6 Se0.4 in 1D Carbon Nanofiber with CS Bonding for High-Performance Flexible Li-S Batteries and Na-S Batteries.[Pubmed:28371449]
Small. 2017 May;13(19).
A one-step synthesis procedure is developed to prepare flexible S0.6 Se0.4 @carbon nanofibers (CNFs) electrode by coheating S0.6 Se0.4 powder with electrospun polyacrylonitrile nanofiber papers at 600 degrees C. The obtained S0.6 Se0.4 @CNFs film can be used as cathode material for high-performance Li-S batteries and room temperature (RT) Na-S batteries directly. The superior lithium/sodium storage performance derives from its rational structure design, such as the chemical bonding between Se and S, the chemical bonding between S0.6 Se0.4 and CNFs matrix, and the 3D CNFs network. This easy one-step synthesis procedure provides a feasible route to prepare electrode materials for high-performance Li-S and RT Na-S batteries.
Biomechanical Assessment of Hip Capsular Repair and Reconstruction Procedures Using a 6 Degrees of Freedom Robotic System.[Pubmed:28371596]
Am J Sports Med. 2017 Jul;45(8):1745-1754.
BACKGROUND: Although acetabular labral repair has been biomechanically validated to improve stability, capsular management of the hip remains a topic of growing interest and controversy. PURPOSE: To biomechanically evaluate the effects of several arthroscopically relevant conditions of the capsule through a robotic, sequential sectioning study. STUDY DESIGN: Controlled laboratory study. METHODS: Ten human cadaveric unilateral hip specimens (mean age, 51.3 years [range, 38-65 years]) from full pelvises were used to test range of motion (ROM) for the intact capsule and for multiple capsular conditions including portal incisions, interportal capsulotomy, interportal capsulotomy repair, T-capsulotomy, T-capsulotomy repair, a large capsular defect, and capsular reconstruction. Hips were biomechanically tested using a 6 degrees of freedom robotic system to assess ROM with applied 5-N.m internal, external, abduction, and adduction rotation torques throughout hip flexion and extension. RESULTS: All capsulotomy procedures (portals, interportal capsulotomy, and T-capsulotomy) created increases in external, internal, adduction, and abduction rotations compared with the intact state throughout the full tested ROM (-10 degrees to 90 degrees of flexion). Reconstruction significantly reduced rotation compared with the large capsular defect state for external rotation at 15 degrees (difference, 1.4 degrees ) and 90 degrees (difference, 1.3 degrees ) of flexion; internal rotation at -10 degrees (difference, 0.4 degrees ), 60 degrees (difference, 0.9 degrees ), and 90 degrees (difference, 1.4 degrees ) of flexion; abduction rotation at -10 degrees (difference, 0.5 degrees ), 15 degrees (difference, 1.1 degrees ), 30 degrees (difference, 1.2 degrees ), 60 degrees (difference, 0.9 degrees ), and 90 degrees (difference, 1.0 degrees ) of flexion; and adduction rotation at 0 degrees (difference, 0.7 degrees ), 15 degrees (difference, 0.8 degrees ), 30 degrees (difference, 0.3 degrees ), and 90 degrees (difference, 0.6 degrees ) of flexion. Repair of T-capsulotomy resulted in significant reductions in rotation compared with the T-capsulotomy condition for abduction rotation at -10 degrees (difference, 0.3 degrees ), 15 degrees (difference, 0.9 degrees ), 30 degrees (difference, 1.3 degrees ), 60 degrees (difference, 1.7 degrees ), and 90 degrees (difference, 1.5 degrees ) of flexion and for internal rotation at -10 degrees (difference, 0.9 degrees ), 60 degrees (difference, 1.5 degrees ), and 90 degrees (difference, 2.6 degrees ) of flexion. Similarly, repair of interportal capsulotomy resulted in significant reductions in abduction (difference, 0.9 degrees ) and internal (difference, 1.4 degrees ) rotations compared with interportal capsulotomy at 90 degrees of flexion. In most cases, however, after the repair procedures, ROM was still increased in comparison with the intact state. CONCLUSION: The results of this study suggest that common hip arthroscopic capsulotomy procedures can result in increases in external, internal, abduction, and adduction rotations throughout a full range (-10 degrees to 90 degrees ) of hip flexion. However, capsular repair and reconstruction succeeded in partially reducing the increased rotational ROM caused by common capsulotomy procedures. Thus, consideration should be allotted toward capsular repair or reconstruction in cases with an increased risk of residual instability. CLINICAL RELEVANCE: Although complete restoration of joint stability may not be fully achieved at time zero, capsular repair and reconstruction may lead to improved patient outcomes by bringing hip rotational movements nearer to normal values in the immediate postoperative period, especially in cases in which extensive capsulotomy is performed.
Efficacy of nutritional supplementation with omega-3 and omega-6 fatty acids in dry eye syndrome: a systematic review of randomized clinical trials.[Pubmed:28371493]
Acta Ophthalmol. 2017 Dec;95(8):e677-e685.
PURPOSE: To critically appraise scientific evidence regarding the efficacy of nutritional supplementation with omega-3 and omega-6 fatty acids for the treatment of dry eye syndrome (DES). METHODS: A systematic review of randomized clinical trials was performed. Two independent reviewers selected and analysed the scientific papers that met inclusion and exclusion criteria. Objective and subjective efficacy outcomes were assessed. RESULTS: The trials involved a total of 2591 patients in fifteen independent studies. All studies were published between 2005 and 2015. The supplements used were mostly omega-3 and omega-6 in different proportions. Subjective improvement was measured using mainly Ocular Surface Disease Index (OSDI) test and Dry Eye Severity Score (DESS) test: significant differences in favour of the experimental group were found in seven of the studies. The objective amelioration was assessed by lacrimal function parameters: Tear break-up time (TBUT) significantly increased in nine studies and Schirmer's test in four studies. CONCLUSION: We observed a discrete improvement in the parameters of tear function. Scientific evidence is not strong enough to systematically recommend the use of omega-3 and omega-6 fatty acids as a standalone treatment of DES independently from its aetiology. However, they could be considered as an effective alternative to topical treatment in patients with DES secondary to certain pathologies.


