HeraclenolCAS# 31575-93-6 |
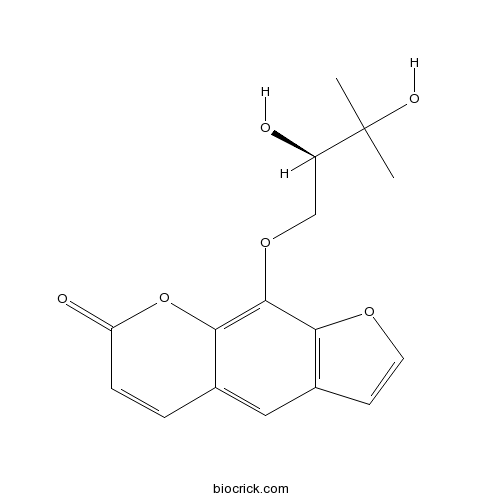
- (-)-Heraclenol
Catalog No.:BCN7682
CAS No.:139079-42-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 31575-93-6 | SDF | Download SDF |
| PubChem ID | 73253 | Appearance | Powder |
| Formula | C16H16O6 | M.Wt | 304.3 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 9-[(2R)-2,3-dihydroxy-3-methylbutoxy]furo[3,2-g]chromen-7-one | ||
| SMILES | CC(C)(C(COC1=C2C(=CC3=C1OC=C3)C=CC(=O)O2)O)O | ||
| Standard InChIKey | FOINLJRVEBYARJ-LLVKDONJSA-N | ||
| Standard InChI | InChI=1S/C16H16O6/c1-16(2,19)11(17)8-21-15-13-10(5-6-20-13)7-9-3-4-12(18)22-14(9)15/h3-7,11,17,19H,8H2,1-2H3/t11-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Heraclenol is a germination inhibitor in the parsley seeds. 2. Heraclenol has anti-inflammatory properties against the ear edema in mice produced by TPA. 3. Heraclenol and heraclenin inhibit the proliferation of melanoma cells and cell cycle at G2/M at concentrations of 0.1-1.0 uM. |
| Targets | Immunology & Inflammation related |

Heraclenol Dilution Calculator

Heraclenol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2862 mL | 16.4312 mL | 32.8623 mL | 65.7246 mL | 82.1558 mL |
| 5 mM | 0.6572 mL | 3.2862 mL | 6.5725 mL | 13.1449 mL | 16.4312 mL |
| 10 mM | 0.3286 mL | 1.6431 mL | 3.2862 mL | 6.5725 mL | 8.2156 mL |
| 50 mM | 0.0657 mL | 0.3286 mL | 0.6572 mL | 1.3145 mL | 1.6431 mL |
| 100 mM | 0.0329 mL | 0.1643 mL | 0.3286 mL | 0.6572 mL | 0.8216 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4EGI-1
Catalog No.:BCC5337
CAS No.:315706-13-9
- PTC-209
Catalog No.:BCC5111
CAS No.:315704-66-6
- JK 184
Catalog No.:BCC3936
CAS No.:315703-52-7
- STF-62247
Catalog No.:BCC4960
CAS No.:315702-99-9
- TC-DAPK 6
Catalog No.:BCC1989
CAS No.:315694-89-4
- Kavain
Catalog No.:BCN8295
CAS No.:3155-48-4
- 1,18-Octadecanediol
Catalog No.:BCN5233
CAS No.:3155-43-9
- Matsukaze-lactone
Catalog No.:BCN7580
CAS No.:3153-73-9
- O-Nornuciferine
Catalog No.:BCN7074
CAS No.:3153-55-7
- 5-Hydroxyseselin
Catalog No.:BCN3428
CAS No.:31525-75-4
- Isobavachin
Catalog No.:BCN5232
CAS No.:31524-62-6
- Sutherlandin trans-p-coumarate
Catalog No.:BCN5231
CAS No.:315236-68-1
- Berberine Sulphate
Catalog No.:BCC8131
CAS No.:316-41-6
- Emetine dihydrochloride
Catalog No.:BCN8307
CAS No.:316-42-7
- Z-Asp(OMe)-OH
Catalog No.:BCC2790
CAS No.:3160-47-2
- Z-Ile-OH
Catalog No.:BCC2593
CAS No.:3160-59-6
- Moroxydine HCl
Catalog No.:BCC4802
CAS No.:3160-91-6
- SCH 442416
Catalog No.:BCC7372
CAS No.:316173-57-6
- Artocarpesin
Catalog No.:BCN8071
CAS No.:3162-09-2
- 5,7-Dihydroxy-6,8-dimethoxyflavone
Catalog No.:BCN5235
CAS No.:3162-45-6
- 9-Anthracenylmethyl acrylate
Catalog No.:BCC8798
CAS No.:31645-34-8
- Palifosfamide
Catalog No.:BCC1833
CAS No.:31645-39-3
- 6-Aminonicotinic acid
Catalog No.:BCC8764
CAS No.:3167-49-5
- Gatifloxacin mesylate
Catalog No.:BCC4225
CAS No.:316819-28-0
Phytotoxic furanocoumarins from the shoots of Semenovia transiliensis.[Pubmed:23157001]
Nat Prod Commun. 2012 Oct;7(10):1327-30.
Discovery of novel, natural herbicides has become important to manage increasing weed resistance to synthetic herbicides and environmental issues. The systematic bioassay-guided fractionation and purification of the methylene chloride/methanol extract of the shoots of Semenovia transiliensis led to the isolation of several phytotoxic compounds. Lactuca sativa L. (lettuce, a dicot) and Agrostis stolonifera L. (bentgrass, a monocot) bioassays were used to identify and isolate the phytotoxic fractions. A number of furanocoumarin compounds isolated from S. transiliensis shoots were phytotoxic to both test species. These included psoralen, isopsoralen, heratomin, isopentenyloxyisobergapten, imperatorin, bergapten, xanthotoxin, heraclenin, and Heraclenol. All the active secondary metabolites isolated from the shoots of S. transiliensis were furanocoumarins. Identification of these was accomplished using mass spectrometry and 1- and 2-dimensional NMR techniques. Phytotoxic activity o f isolated compounds w a s evaluated in a dose-response manner from 0.3 to 1000 microM. Ingeneral, all of the compounds were more active on A. stolonifera than L. sativa. Bergaptin and xanthotoxin were the most active of the compounds, with moderate activity at 100 microM. Imperatorin and xanthotoxin inhibited growth of Lemna paucicostata Hegelm. by 50% at 29 and 60 microM, respectively. Our results show that S. transiliensis is rich in furanocoumarins, which are probably involved in various aspects of the chemical ecology of the species. Unfortunately, the general cytotoxicity of furanocoumarins makes them an unlikely candidate for pesticide discovery.
In vitro and in vivo antiproliferative effect of a combination of ultraviolet-A and alkoxy furocoumarins isolated from Umbelliferae medicinal plants, in melanoma cells.[Pubmed:23802687]
Photochem Photobiol. 2013 Sep-Oct;89(5):1216-25.
We examined the effects of six furocoumarins with alkoxy groups at the C-5 or C-8 position isolated from Umbelliferae medicinal plants on cell proliferation, and their mechanisms of action against B16F10 melanoma cells or in melanin-possessing hairless mice implanted with B16F10 cells, under UVA irradiation. Three furocoumarins with an alkoxy group at C-5, isoimperatorin (1), oxypeucedanin (2) and oxypeucedanin hydrate (3), showed antiproliferative activity and caused G2/M arrest at concentrations of 0.1-10.0 mum. Furthermore, three furocoumarins with an alkoxy group at C-8, imperatorin (4), heraclenin (5) and Heraclenol (6), inhibited the proliferation of melanoma cells and cell cycle at G2/M at concentrations of 0.1-1.0 mum. UVA plus 1, 2, 3, 4 and 6 reduced tumor growth and final tumor weight in B16F10-bearing mice at a dose of 0.3, 0.5 or 1.0 mg kg(-1) (intraperitoneal injection). UVA plus 1, 3 and 6 increased Chk1 phosphorylation and reduced cdc2 (Thr 161) phosphorylation in melanoma cells. We suggest that the antitumor actions of UVA plus furocoumarins with an alkoxy group at C-5 or C-8 were due to G2/M arrest of the cell cycle by an increase in phosphor-Chk1 and decrease in phospho-cdc2.
Anti-inflammatory activity of coumarins from Decatropis bicolor on TPA ear mice model.[Pubmed:10821059]
Planta Med. 2000 Apr;66(3):279-81.
From the aerial parts of Decatropis bicolor, heraclenin (1), seselin (2), psoralen (3), imperatorin (4), skimmianine (5), and Heraclenol (6), were isolated. This is the first time that coumarin-like compounds are isolated from Decatropis genus. The anti-inflammatory properties of compounds 1-6 were examined against the ear edema in mice produced by TPA. The results suggest that the anti-inflammatory activity of each compound depends of its individual substitution on the aromatic ring rather than the coumarin skeleton itself.
Isolation, cytotoxicity evaluation and HPLC-quantification of the chemical constituents from Prangos pabularia.[Pubmed:25314269]
PLoS One. 2014 Oct 14;9(10):e108713.
Phytochemical analysis of the dichloromethane:methanol (1:1) extract of root parts of Prangos pabularia led to the isolation of twelve cytotoxic constituents, viz., 6-hydroxycoumarin (1), 7-hydroxycoumarin (2), Heraclenol-glycoside (3), xanthotoxol (4), Heraclenol (5), oxypeucedanin hydrate (6), 8-((3,3-dimethyloxiran-2-yl)methyl)-7-methoxy-2H-chromen-2-one (7), oxypeucedanin hydrate monoacetate (8), xanthotoxin (9), 4-((2-hydroxy-3-methylbut-3-en-1-yl)oxy)-7H-furo[3,2-g]chromen-7-one (10), imperatorin (11) and osthol (12). The isolates were identified using spectral techniques in the light of literature. 3-(4,5-dimethyl thiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) cytotoxicity screening of the isolated constituents was carried out against six human cancer cell lines including lung (A549 and NCI-H322), epidermoid carcinoma (A431), melanoma (A375), prostate (PC-3) and Colon (HCT-116) cell lines. Osthol (12) exhibited the highest cytotoxicity with IC50 values of 3.2, 6.2, 10.9, 14.5, 24.8, and 30.2 microM against epidermoid carcinoma (A431), melanoma (A375), lung (NCI-H322), lung (A549), prostate (PC-3) and colon (HCT-116) cell lines respectively. Epidermoid carcinoma cell line A431 was sensitive to most of the compounds followed by lung (A549) cancer cell line. Finally a simple and reliable HPLC method was developed (RP-HPLC-DAD) and validated for the simultaneous quantification of these cytotoxic constituents in Prangos pabularia. The extract was analyzed using a reversed-phase Agilent ZORBAX eclipse plus column C18 (4.6x250 mm, 5 microm) at 250 nm wavelength using a gradient water-methanol solvent system at a flow rate of 0.8 ml/min. The RP-HPLC method is validated in terms of recovery, linearity, accuracy and precision (intra and inter-day validation). This method, because of shorter analysis time, makes it valuable for the commercial quality control of Prangos pabularia extracts and its future pharmaceutical preparations.


