TACAGABA uptake inhibitor. Also GABAA agonist and substrate for GABA-T CAS# 38090-53-8 |
- FLAG tag Peptide
Catalog No.:BCC2562
CAS No.:98849-88-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 38090-53-8 | SDF | Download SDF |
| PubChem ID | 5310987 | Appearance | Powder |
| Formula | C4H7NO2 | M.Wt | 101.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
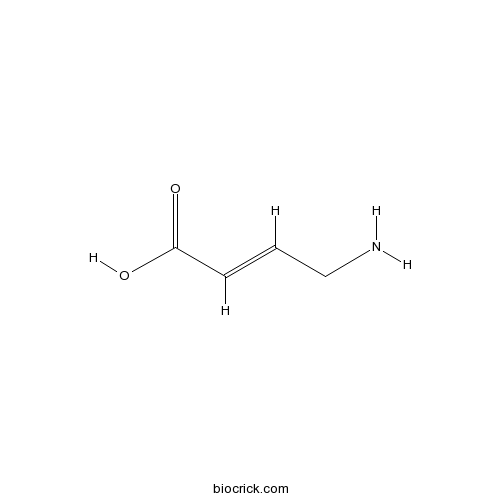
| Chemical Name | (E)-4-aminobut-2-enoic acid | ||
| SMILES | C(C=CC(=O)O)N | ||
| Standard InChIKey | FMKJUUQOYOHLTF-OWOJBTEDSA-N | ||
| Standard InChI | InChI=1S/C4H7NO2/c5-3-1-2-4(6)7/h1-2H,3,5H2,(H,6,7)/b2-1+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | The trans-isomer of CACA. Potent GABAA agonist, GABA uptake inhibitor and substrate for GABA-T. Also GABAA-ρ agonist. |

TACA Dilution Calculator

TACA Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 9.8912 mL | 49.456 mL | 98.912 mL | 197.8239 mL | 247.2799 mL |
| 5 mM | 1.9782 mL | 9.8912 mL | 19.7824 mL | 39.5648 mL | 49.456 mL |
| 10 mM | 0.9891 mL | 4.9456 mL | 9.8912 mL | 19.7824 mL | 24.728 mL |
| 50 mM | 0.1978 mL | 0.9891 mL | 1.9782 mL | 3.9565 mL | 4.9456 mL |
| 100 mM | 0.0989 mL | 0.4946 mL | 0.9891 mL | 1.9782 mL | 2.4728 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bosutinib (SKI-606)
Catalog No.:BCC1167
CAS No.:380843-75-4
- Pterolactam
Catalog No.:BCN5435
CAS No.:38072-88-7
- [(pF)Phe4]Nociceptin(1-13)NH2
Catalog No.:BCC5778
CAS No.:380620-88-2
- CMPDA
Catalog No.:BCC6151
CAS No.:380607-77-2
- DQP 1105
Catalog No.:BCC6205
CAS No.:380560-89-4
- 187-1, N-WASP inhibitor
Catalog No.:BCC5866
CAS No.:380488-27-7
- alpha-Epoxydihydroartemisinic acid
Catalog No.:BCN5434
CAS No.:380487-65-0
- NBMPR
Catalog No.:BCC7516
CAS No.:38048-32-7
- (+)-columbianetin
Catalog No.:BCN2331
CAS No.:3804-70-4
- Tenovin-1
Catalog No.:BCC2239
CAS No.:380315-80-0
- Sulfamonomethoxine sodium
Catalog No.:BCC9157
CAS No.:38006-08-5
- 2-Amino-4'-fluorobenzophenone
Catalog No.:BCC8530
CAS No.:3800-06-4
- Perampanel
Catalog No.:BCC1847
CAS No.:380917-97-5
- Streptomycin sulfate
Catalog No.:BCC4851
CAS No.:3810-74-0
- Brevianamide F
Catalog No.:BCN6452
CAS No.:38136-70-8
- 28-Hydroxy-3-oxoolean-12-en-29-oic acid
Catalog No.:BCN1449
CAS No.:381691-22-1
- Bephenium Hydroxynaphthoate
Catalog No.:BCC3735
CAS No.:3818-50-6
- 7,8-Dihydroxyflavone
Catalog No.:BCC6072
CAS No.:38183-03-8
- Sulindac
Catalog No.:BCC4861
CAS No.:38194-50-2
- Adrenosterone
Catalog No.:BCC4061
CAS No.:382-45-6
- Bacopaside II
Catalog No.:BCC8125
CAS No.:382146-66-9
- Coumarin VI
Catalog No.:BCN7833
CAS No.:38215-36-0
- Pyroxamide
Catalog No.:BCC2424
CAS No.:382180-17-8
- Filixic acid ABA
Catalog No.:BCN6330
CAS No.:38226-84-5
The calcineurin dependent transcription factor TacA is involved in development and the stress response of Dictyostelium discoideum.[Pubmed:22944283]
Eur J Cell Biol. 2012 Oct;91(10):789-99.
Calcineurin is an important signalling protein in a plethora of Ca(2+)-regulated cellular processes. In contrast to what is known about the function of calcineurin in various organisms, information on calcineurin substrates is still limited. Here we describe the identification and characterisation of the transcription factor activated by calcineurin (TACA) in the model organism Dictyostelium discoideum. TACA is a putative zinc-finger transcription factor orthologue of yeast Crz1. In resting unstimulated cells the protein is located in the cytosol and translocates to the nucleus in a calcineurin-dependent manner after Ca(2+)-stimulation. Nuclear export of TACA is partially dependent on GskA, the Dictyostelium orthologue of mammalian GSK3. The expression of TACA is developmentally regulated with its kinetics roughly paralleling calcineurin regulation. Silencing of TACA via RNAi leads to developmental defects and dysregulation of developmentally regulated and Ca(2+)-regulated marker genes. Additionally, TACA is involved in the stress response of D. discoideum during development in a separate pathway to the well-known stress response in Dictyostelium via STATc. Finally we provide evidence that TACA is not only an orthologue of yeast Crz1 but also functionally related to mammalian NFAT.
Decreased cellulase and xylanase production in the fungus Talaromyces cellulolyticus by disruption of tacA and tctA genes, encoding putative zinc finger transcriptional factors.[Pubmed:25627293]
Appl Biochem Biotechnol. 2015 Mar;175(6):3218-29.
Talaromyces cellulolyticus (formerly Acremonium cellulolyticus) is one of the important strains for industrial cellulase production. An understanding of the control of cellulase gene expression in T. cellulolyticus is insufficient because only a few transcriptional factors related to cellulase gene expression have been identified. In the present study, we disrupted seven putative transcription regulator genes that showed similarity with cellulase or hemicellulase regulator genes in other filamentous fungi and investigated whether these genes are related to cellulase and xylanase production. Among the seven genes, five (tclA, tbgA, tlaA, tmcA, tclB2) had a smaller effect on cellulase and xylanase activities when culturing with cellulose. On the other hand, disruption of TACA and tctA, which are respectively homologues of ace1 (repressor of cellulase) and ctf1 (inducer of cutinase), led to a decrease in cellulase and hemicellulase production due to effects at both the enzymatic and transcriptional levels, indicating that TACA and tctA have positive roles in cellulase and xylanase production in T. cellulolyticus. These results suggest that cellulase and xylanase gene regulation in T. cellulolyticus differs from that in other filamentous fungi and imply that unknown transcriptional mechanisms function in T. cellulolyticus.
Analogues of gamma-aminobutyric acid (GABA) and trans-4-aminocrotonic acid (TACA) substituted in the 2 position as GABAC receptor antagonists.[Pubmed:9422798]
Br J Pharmacol. 1997 Dec;122(8):1551-60.
1. gamma-Aminobutyric acid (GABA) and trans-4-aminocrotonic acid (TACA) have been shown to activate GABAC receptors. In this study, a range of C2, C3, C4 and N-substituted GABA and TACA analogues were examined for activity at GABAC receptors. 2. The effects of these compounds were examined by use of electrophysiological recording from Xenopus oocytes expressing the human rho 1 subunit of GABAC receptors with the two-electrode voltage-clamp method. 3. trans-4-Amino-2-fluorobut-2-enoic acid was found to be a potent agonist (KD = 2.43 microM). In contrast, trans-4-amino-2-methylbut-2-enoic acid was found to be a moderately potent antagonist (IC50 = 31.0 microM and KB = 45.5 microM). These observations highlight the possibility that subtle structural substitutions may change an agonist into an antagonist. 4. 4-Amino-2-methylbutanoic acid (KD = 189 microM), 4-amino-2-methylenebutanoic acid (KD = 182 microM) and 4-amino-2-chlorobutanoic acid (KD = 285 microM) were weak partial agonists. The intrinsic activities of these compounds were 12.1%, 4.4% and 5.2% of the maximal response of GABA, respectively. These compounds more effectively blocked the effects of the agonist, GABA, giving rise to KB values of 53 microM and 101 microM, respectively. 5. The sulphinic acid analogue of GABA, homohypotaurine, was found to be a potent partial agonist (KD = 4.59 microM, intrinsic activity 69%). 6. It was concluded that substitution of a methyl or a halo group in the C2 position of GABA or TACA is tolerated at GABAC receptors. However, there was dramatic loss of activity when these groups were substituted at the C3, C4 and nitrogen positions of GABA and TACA. 7. Molecular modelling studies on a range of active and inactive compounds indicated that the agonist/competitive antagonist binding site of the GABAC receptor may be smaller than that of the GABAA and GABAB receptors. It is suggested that only compounds that can attain relatively flat conformations may bind to the GABAC receptor agonist/competitive antagonist binding site.
Trans-4-aminocrotonic acid (TACA), a potent agonist of GABA(A) and GABA(C) receptors, shows a proconvulsant action in the electroconvulsive threshold test in mice.[Pubmed:15849386]
Pharmacol Rep. 2005 Jan-Feb;57(1):121-3.
In the present study, we evaluated TACA (a potent agonist of GABA(A) and GABA(C) receptors) in the electroconvulsive threshold test in mice. Surprisingly, TACA (at 15 and 25 mg/kg) significantly decreased the threshold. The highest ineffective dose of TACA was estimated as 10 mg/kg. The obtained results indicate that the drug enhancing GABAergic transmission may possess proconvulsant properties in the electroconvulsive test. Such effect was completely opposite to our primary assumption and expectance.
GABAc receptors: relatively simple transmitter -gated ion channels?[Pubmed:8885697]
Trends Pharmacol Sci. 1996 Sep;17(9):319-23.
The inhibitory neurotransmitter, GABA, activates a variety of receptors in all areas of the CNS. Two major subtypes of GABA receptors are well known: (1) GABAA receptors are ligand-gated Cl- channels that consist of a heteromeric mixture of protein subunits forming a pentameric structure, and (2) GABAB receptors couple to Ca2+ and K+ channels via G proteins and second messengers. Here, Graham Johnston discusses evidence for a third major subclass of GABA receptors. GABAC receptors appear to be relatively simple ligand-gated Cl- channels with a distinctive pharmacology, in that they are not blocked by bicuculline and not modulated by barbiturates, benzodiazepines or neuroactive steroids. Compared with GABAA receptors, GABAC receptors are activated at lower concentrations of GABA and are less liable to desensitization. In addition, their channels open for a longer time. The pharmacology of these novel subtypes of GABA receptors may yield important therapeutic agents.


