NBMPREquilibrative nucleoside transporter 1 (ENT1) inhibitor CAS# 38048-32-7 |
- Limonin
Catalog No.:BCN6057
CAS No.:1180-71-8
- Fosamprenavir Calcium Salt
Catalog No.:BCC1581
CAS No.:226700-81-8
- HIV-1 integrase inhibitor
Catalog No.:BCC1618
CAS No.:544467-07-4
- BMS-626529
Catalog No.:BCC1427
CAS No.:701213-36-7
- HIV-1 integrase inhibitor 2
Catalog No.:BCC1619
CAS No.:957890-42-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 38048-32-7 | SDF | Download SDF |
| PubChem ID | 5129 | Appearance | Powder |
| Formula | C17H17N5O6S | M.Wt | 419.41 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
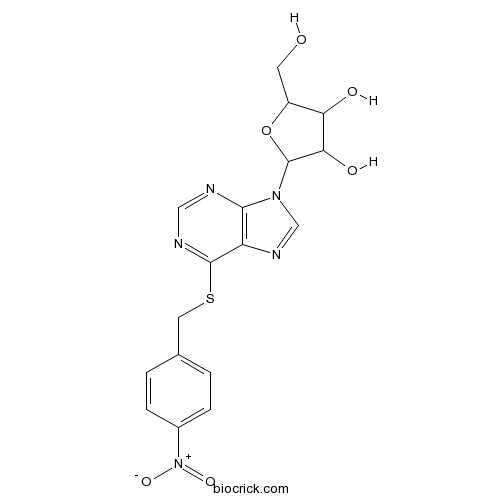
| Chemical Name | 2-(hydroxymethyl)-5-[6-[(4-nitrophenyl)methylsulfanyl]purin-9-yl]oxolane-3,4-diol | ||
| SMILES | C1=CC(=CC=C1CSC2=NC=NC3=C2N=CN3C4C(C(C(O4)CO)O)O)[N+](=O)[O-] | ||
| Standard InChIKey | DYCJFJRCWPVDHY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H17N5O6S/c23-5-11-13(24)14(25)17(28-11)21-8-20-12-15(21)18-7-19-16(12)29-6-9-1-3-10(4-2-9)22(26)27/h1-4,7-8,11,13-14,17,23-25H,5-6H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Equilibrative nucleoside transporter 1 (ENT1) inhibitor (Ki values are 0.4 and 2800 nM for hENT1 and hENT2 respectively). |

NBMPR Dilution Calculator

NBMPR Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3843 mL | 11.9215 mL | 23.843 mL | 47.686 mL | 59.6075 mL |
| 5 mM | 0.4769 mL | 2.3843 mL | 4.7686 mL | 9.5372 mL | 11.9215 mL |
| 10 mM | 0.2384 mL | 1.1922 mL | 2.3843 mL | 4.7686 mL | 5.9608 mL |
| 50 mM | 0.0477 mL | 0.2384 mL | 0.4769 mL | 0.9537 mL | 1.1922 mL |
| 100 mM | 0.0238 mL | 0.1192 mL | 0.2384 mL | 0.4769 mL | 0.5961 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (+)-columbianetin
Catalog No.:BCN2331
CAS No.:3804-70-4
- Tenovin-1
Catalog No.:BCC2239
CAS No.:380315-80-0
- Sulfamonomethoxine sodium
Catalog No.:BCC9157
CAS No.:38006-08-5
- 2-Amino-4'-fluorobenzophenone
Catalog No.:BCC8530
CAS No.:3800-06-4
- LM 22A4
Catalog No.:BCC6239
CAS No.:37988-18-4
- Geranylacetone
Catalog No.:BCN7567
CAS No.:3796-70-1
- tenofovir diphosphate
Catalog No.:BCC6447
CAS No.:166403-66-3
- Tenofovir Alafenamide Fumarate
Catalog No.:BCC8067
CAS No.:379270-38-9
- Tenofovir alafenamide
Catalog No.:BCC8066
CAS No.:379270-37-8
- Saracatinib (AZD0530)
Catalog No.:BCC1166
CAS No.:379231-04-6
- Cimifugin
Catalog No.:BCN5433
CAS No.:37921-38-3
- Jolkinolide B
Catalog No.:BCN2391
CAS No.:37905-08-1
- alpha-Epoxydihydroartemisinic acid
Catalog No.:BCN5434
CAS No.:380487-65-0
- 187-1, N-WASP inhibitor
Catalog No.:BCC5866
CAS No.:380488-27-7
- DQP 1105
Catalog No.:BCC6205
CAS No.:380560-89-4
- CMPDA
Catalog No.:BCC6151
CAS No.:380607-77-2
- [(pF)Phe4]Nociceptin(1-13)NH2
Catalog No.:BCC5778
CAS No.:380620-88-2
- Pterolactam
Catalog No.:BCN5435
CAS No.:38072-88-7
- Bosutinib (SKI-606)
Catalog No.:BCC1167
CAS No.:380843-75-4
- TACA
Catalog No.:BCC6564
CAS No.:38090-53-8
- Perampanel
Catalog No.:BCC1847
CAS No.:380917-97-5
- Streptomycin sulfate
Catalog No.:BCC4851
CAS No.:3810-74-0
- Brevianamide F
Catalog No.:BCN6452
CAS No.:38136-70-8
- 28-Hydroxy-3-oxoolean-12-en-29-oic acid
Catalog No.:BCN1449
CAS No.:381691-22-1
Constrained NBMPR analogue synthesis, pharmacophore mapping and 3D-QSAR modeling of equilibrative nucleoside transporter 1 (ENT1) inhibitory activity.[Pubmed:18289860]
Bioorg Med Chem. 2008 Apr 1;16(7):3848-65.
Conformationally constrained analogue synthesis was undertaken to aid in pharmacophore mapping and 3D-QSAR analysis of nitrobenzylmercaptopurine riboside (NBMPR) congeners as equilibriative nucleoside transporter 1 (ENT1) inhibitors. In our previous study [J. Med. Chem. 2003, 46, 831-837], novel regioisomeric nitro-1,2,3,4-tetrahydroisoquinoline conformationally constrained analogues of NBMPR were synthesized and evaluated as ENT1 ligands. 7-NO(2)-1,2,3,4-Tetrahydroisoquino-2-yl purine riboside was identified as the analogue with the nitro group in the best orientation at the NBMPR binding site of ENT1. In the present study, further conformational constraining was introduced by synthesizing 5'-O,8-cyclo derivatives. The flow cytometrically determined binding affinities indicated that the additional 5'-O,8-cyclo constraining was unfavorable for binding to the ENT1 transporter. The structure-activity relationship (SAR) acquired was applied to pharmacophore mapping using the PHASE program. The best pharmacophore hypothesis obtained embodied an anti-conformation with three hydrogen-bond acceptors, one hydrophobic center, and two aromatic rings involving the 3'-OH, 4'-oxygen, the NO(2) group, the benzyl phenyl and the imidazole and pyrimidine portions of the purine ring, respectively. A PHASE 3D-QSAR model derived with this pharmacophore yielded an r(2) of 0.916 for four (4) PLS components, and an excellent external test set predictive r(2) of 0.78 for 39 compounds. This pharmacophore was used for molecular alignment in a comparative molecular field analysis (CoMFA) 3D-QSAR study that also afforded a predictive model with external test set validation predictive r(2) of 0.73. Thus, although limited, this study suggests that the bioactive conformation for NBMPR at the ENT1 transporter could be anti. The study has also suggested an ENT1 inhibitory pharmacophore, and established a predictive CoMFA 3D-QSAR model that might be useful for novel ENT1 inhibitor discovery and optimization.
Localization of the NBMPR-sensitive equilibrative nucleoside transporter, ENT1, in the rat dorsal root ganglion and lumbar spinal cord.[Pubmed:16226730]
Brain Res. 2005 Oct 19;1059(2):129-38.
ENT1 is an equilibrative nucleoside transporter that enables trans-membrane bi-directional diffusion of biologically active purines such as adenosine. In spinal cord dorsal horn and in sensory afferent neurons, adenosine acts as a neuromodulator with complex pro- and anti-nociceptive actions. Although uptake and release mechanisms for adenosine are believed to exist in both the dorsal horn and sensory afferent neurons, the expression profile of specific nucleoside transporter subtypes such as ENT1 is not established. In this study, immunoblot analysis with specific ENT1 antibodies (anti-rENT1(227-290) or anti-hENT1(227-290)) was used to reveal the expression of ENT1 protein in tissue homogenates of either adult rat dorsal horn or dorsal root ganglia (DRG). Immunoperoxidase labeling with ENT1 antibodies produced specific staining in dorsal horn which was concentrated over superficial laminae, especially the substantia gelatinosa (lamina II). Immunofluorescence double-labeling revealed a punctate pattern for ENT1 closely associated, in some instances, with cell bodies of either neurons (confirmed with NeuN) or glia (confirmed with CNPase). Electron microscopy analysis of ENT1 expression in lamina II indicated its presence within pre- and post-synaptic elements, although a number of other structures, including myelinated and unmyelinated, axons were also labeled. In sensory ganglia, ENT1 was localized to a high proportion of cell bodies of all sizes that co-expressed substance P, IB4 or NF, although ENT1 was most highly expressed in the peptidergic population. These data provide the first detailed account of the expression and cellular distribution of ENT1 in rat dorsal horn and sensory ganglia. The functional significance of ENT1 expression with regard to the homeostatic regulation of adenosine at synapses remains to be established.
Mutation of leucine-92 selectively reduces the apparent affinity of inosine, guanosine, NBMPR [S6-(4-nitrobenzyl)-mercaptopurine riboside] and dilazep for the human equilibrative nucleoside transporter, hENT1.[Pubmed:14759222]
Biochem J. 2004 May 15;380(Pt 1):131-7.
We developed a yeast-based assay for selection of hENT1 (human equilibrative nucleoside transporter 1) mutants that have altered affinity for hENT1 inhibitors and substrates. In this assay, expression of hENT1 in a yeast strain deficient in adenine biosynthesis (ade2) permits yeast growth on a plate lacking adenine but containing adenosine, a hENT1 substrate. This growth was prevented when inhibitors of hENT1 [e.g. NBMPR [S6-(4-nitrobenzyl)-mercaptopurine riboside], dilazep or dipyridamole] were included in the media. To identify hENT1 mutants resistant to inhibition by these compounds, hENT1 was randomly mutagenized and introduced into this strain. Mutation(s) that allowed growth of yeast cells in the presence of these inhibitors were then identified and characterized. Mutants harbouring amino acid changes at Leu92 exhibited resistance to NBMPR and dilazep but not dipyridamole. The IC50 values of NBMPR and dilazep for [3H]adenosine transport by one of these mutants L92Q (Leu92-->Gln) were approx. 200- and 4-fold greater when compared with the value for the wild-type hENT1, whereas that for dipyridamole remained unchanged. Additionally, when compared with the wild-type transporter, [3H]adenosine transport by L92Q transporter was significantly resistant to inhibition by inosine and guanosine but not by adenosine or pyrimidines. The Km value for inosine transport was approx. 4-fold greater for the L92Q mutant (260+/-16 mM) when compared with the wild-type transporter (65+/-7.8 mM). We have identified for the first time an amino acid residue (Leu92) of hENT1 that, when mutated, selectively alters the affinity of hENT1 to transport the nucleosides inosine and guanosine and its sensitivity to the inhibitors NBMPR and dilazep.
CoMFA and CoMSIA 3D-QSAR studies on S(6)-(4-nitrobenzyl)mercaptopurine riboside (NBMPR) analogs as inhibitors of human equilibrative nucleoside transporter 1 (hENT1).[Pubmed:19091561]
Bioorg Med Chem Lett. 2009 Jan 15;19(2):314-8.
3D-QSAR (CoMFA and CoMSIA) studies were performed on human equlibrative nucleoside transporter (hENT1) inhibitors displaying K(i) values ranging from 10,000 to 0.7nM. Both CoMFA and CoMSIA analysis gave reliable models with q2 values >0.50 and r2 values >0.92. The models have been validated for their stability and robustness using group validation and bootstrapping techniques and for their predictive abilities using an external test set of nine compounds. The high predictive r2 values of the test set (0.72 for CoMFA model and 0.74 for CoMSIA model) reveals that the models can prove to be a useful tool for activity prediction of newly designed nucleoside transporter inhibitors. The CoMFA and CoMSIA contour maps identify features important for exhibiting good binding affinities at the transporter, and can thus serve as a useful guide for the design of potential equilibrative nucleoside transporter inhibitors.
Synthesis, flow cytometric evaluation, and identification of highly potent dipyridamole analogues as equilibrative nucleoside transporter 1 inhibitors.[Pubmed:17636949]
J Med Chem. 2007 Aug 9;50(16):3906-20.
Dipyridamole (Persantine) is a clinically used vasodilator with equilibrative nucleoside transporters 1 and 2 (ENT1 and ENT2) inhibitory activity albeit less potent than the prototype ENT1 inhibitor nitrobenzylmercaptopurine riboside (NBMPR). Dipyridamole is a good candidate for further exploration because it is a non-nucleoside and has a proven record of safe use in humans. A series of dipyridamole analogues were synthesized with systematic modification and evaluated as ENT1 inhibitors by flow cytometry. Compounds with much higher potency were identified, the best being 2,6-bis(diethanolamino)-4,8-diheptamethyleneiminopyrimido[5,4-d]pyrimidine (13) with a K(i) of 0.49 nM compared to a K(i) of 308 nM for dipyridamole. Compound 13 is similar in potency to the prototype potent ENT1 inhibitor NBMPR (0.43 nM). For the first time, a dipyridamole analogue has been identified that is equipotent with NBMPR. The SAR indicated that diethanolamine substituted analogues were more active than monoethanolamine compounds. Also, free hydroxyl groups are not essential for activity.
Human equilibrative nucleoside transporter 1, as a predictor of 5-fluorouracil resistance in human pancreatic cancer.[Pubmed:17695509]
Anticancer Res. 2007 Jul-Aug;27(4B):2241-9.
BACKGROUND: The purpose of this study was to find a novel biomarker to predict 5-fluorouracil (5-FU) or gemcitabine (2',2'-difluoro-deoxycytidine) sensitivity in pancreatic cancer. MATERIALS AND METHODS: The relationship between 5-FU and gemcitabine sensitivity and the mRNA levels of human equilibrative nucleoside transporter 1 (hENT1), thymidylate synthase (TS) and dihydropyrimidine dehydrogenase (DPD) was investigated using seven types of human pancreatic carcinoma cell line (AsPC1, BxPC3, MiaPaCa-2, PSN1, Panc1, PCI6, and KMP-4). Quantitative mRNA expression was measured by LightCycler. A [3H] gemcitabine cellular uptake assay was performed to examine the inhibition of hENT1 by nitrobenzylmercaptoprine ribonucleoside (NBMPR). RESULTS: The expression levels of hENT1 mRNA significantly correlated with the IC50 value of 5-FU in all seven lines and also correlated with gemcitabine resistance in six lines (except AsPC1). No significant association was observed between TS or DPD mRNA levels and 5-FU sensitivity. In the PSN1 cells, [3H] gemcitabine uptake via hENT1 was significantly inhibited by NBMPR, and 5-FU sensitivity was significantly increased when the cells were pretreated with NBMPR. CONCLUSION: Our results suggest that hENT1 plays an important role in 5-FU resistance and that hENT1 mRNA levels might be a useful marker to predict 5-FU sensitivity in pancreatic cancer.
Kinetic and pharmacological properties of cloned human equilibrative nucleoside transporters, ENT1 and ENT2, stably expressed in nucleoside transporter-deficient PK15 cells. Ent2 exhibits a low affinity for guanosine and cytidine but a high affinity for inosine.[Pubmed:10722669]
J Biol Chem. 2000 Mar 24;275(12):8375-81.
We stably transfected the cloned human equilibrative nucleoside transporters 1 and 2 (hENT1 and hENT2) into nucleoside transporter-deficient PK15NTD cells. Although hENT1 and hENT2 are predicted to be 50-kDa proteins, hENT1 runs as 40 kDa and hENT2 migrates as 50 and 47 kDa on SDS-polyacrylamide gel electrophoresis. Peptide N-glycosidase F and endoglycosidase H deglycosylate hENT1 to 37 kDa and hENT2 to 45 kDa. With hENT1 being more sensitive, there is a 7000-fold and 71-fold difference in sensitivity to nitrobenzylthioinosine (NBMPR) (IC(50), 0.4 +/- 0.1 nM versus 2.8 +/- 0.3 microM) and dipyridamole (IC(50), 5.0 +/- 0.9 nM versus 356 +/- 13 nM), respectively. [(3)H]NBMPR binds to ENT1 cells with a high affinity K(d) of 0.377 +/- 0.098 nM, and each ENT1 cell has 34,000 transporters with a turnover number of 46 molecules/s for uridine. Although both transporters are broadly selective, hENT2 is a generally low affinity nucleoside transporter with 2.6-, 2.8-, 7. 7-, and 19.3-fold lower affinity than hENT1 for thymidine, adenosine, cytidine, and guanosine, respectively. In contrast, the affinity of hENT2 for inosine is 4-fold higher than hENT1. The nucleobase hypoxanthine inhibits [(3)H]uridine uptake by hENT2 but has minimal effect on hENT1. Taken together, these results suggest that hENT2 might be important in transporting adenosine and its metabolites (inosine and hypoxanthine) in tissues such as skeletal muscle where ENT2 is predominantly expressed.


