(+)-columbianetinCAS# 3804-70-4 |
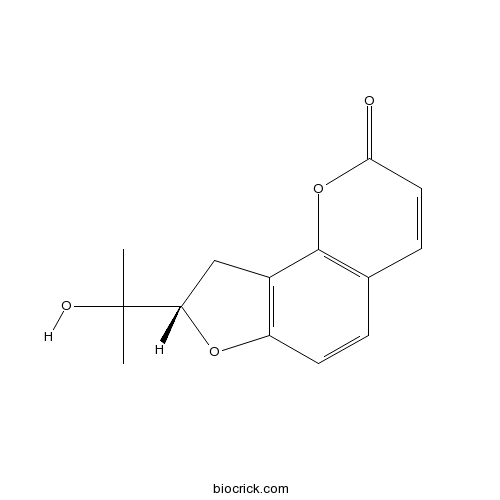
- Columbianetin
Catalog No.:BCN8502
CAS No.:1147-29-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3804-70-4 | SDF | Download SDF |
| PubChem ID | 92201 | Appearance | White powder |
| Formula | C14H14O4 | M.Wt | 246.26 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (8S)-8-(2-hydroxypropan-2-yl)-8,9-dihydrofuro[2,3-h]chromen-2-one | ||
| SMILES | CC(C)(C1CC2=C(O1)C=CC3=C2OC(=O)C=C3)O | ||
| Standard InChIKey | YRAQEMCYCSSHJG-NSHDSACASA-N | ||
| Standard InChI | InChI=1S/C14H14O4/c1-14(2,16)11-7-9-10(17-11)5-3-8-4-6-12(15)18-13(8)9/h3-6,11,16H,7H2,1-2H3/t11-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Columbianetin is a new phytoalexin associated with celery (Apium graveolens) resistance to pathogens during storage, it also has antifungal activity. Columbianetin has anti-inflammatory effects, it promotes histamine release, and inhibits the histamine release by substance P, suggests that it may be helpful in regulating mast cell-mediated allergic inflammatory responses. (2'S)-columbianetin can be effectively used to protect keratinocytes from UVB induced damage. |
| Targets | COX | IL Receptor | TNF-α | NF-kB | NOS | ROS | MAPK | AP-1 |
| In vitro | Protective effect of (2'S)-columbianetin from Corydalis heterocarpa on UVB-induced keratinocyte damage.[Pubmed: 22321694 ]J Photochem Photobiol B. 2012 Apr 2;109:20-7.A salt tolerant plant, Corydalis heterocarpa has been used as a folk medicine to treat travail and spasm. Recent studies have also reported antioxidant and antiinflammatory activities of compounds isolated from C. heterocarpa. In this study, the protective effect of (2'S)-Columbianetin isolated from C. heterocarpa on UVB-induced human keratinocyte (HaCaT) damage was investigated. Columbianetin, a phytoalexin associated with celery resistance to pathogens during storage.[Reference: WebLink]Phytochemistry, 1995, 39(6):1347-50.Columbianetin, rather than psoralens, was found to be a new phytoalexin associated with celery (Apium graveolens) resistance to pathogens during storage. |
| In vivo | The pharmacokinetics and oral bioavailability studies of columbianetin in rats after oral and intravenous administration.[Pubmed: 23994338]J Ethnopharmacol. 2013 Oct 28;150(1):175-80.The roots of Angelica pubescens Maxim. f. biserrata Shan et Yuan (RAP) has been used as Traditional Chinese medicine to treat rheumatic disease in China since ancient times, but its action mechanisms was not well understood. Columbianetin is one of the main active constituents isolated from RAP, which has been shown to have various biological activities, but the absorption characteristics and oral bioavailability dose proportionality of Columbianetin in vivo were not studied.
|
| Kinase Assay | Anti-inflammatory effect of Columbianetin on activated human mast cells.[Pubmed: 19483309]Biol Pharm Bull. 2009 Jun;32(6):1027-31.We isolated the active compound, Columbianetin. Anti-inflammatory effect of Columbianetin has been reported but the precise effects of Columbianetin in experimental models have remained unknown. |
| Structure Identification | Biomed Chromatogr. 2016 Feb;30(2):256-62.Tissue distribution study of columbianadin and its active metabolite columbianetin in rats.[Pubmed: 26115176]Columbianadin, one of the main bioactive constituents of the roots of Angelica pubescens Maxim. f. biserrata Shan et Yuan, has been found to possess obvious pharmacological effects in previous studies. |

(+)-columbianetin Dilution Calculator

(+)-columbianetin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0607 mL | 20.3037 mL | 40.6075 mL | 81.215 mL | 101.5187 mL |
| 5 mM | 0.8121 mL | 4.0607 mL | 8.1215 mL | 16.243 mL | 20.3037 mL |
| 10 mM | 0.4061 mL | 2.0304 mL | 4.0607 mL | 8.1215 mL | 10.1519 mL |
| 50 mM | 0.0812 mL | 0.4061 mL | 0.8121 mL | 1.6243 mL | 2.0304 mL |
| 100 mM | 0.0406 mL | 0.203 mL | 0.4061 mL | 0.8121 mL | 1.0152 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tenovin-1
Catalog No.:BCC2239
CAS No.:380315-80-0
- Sulfamonomethoxine sodium
Catalog No.:BCC9157
CAS No.:38006-08-5
- 2-Amino-4'-fluorobenzophenone
Catalog No.:BCC8530
CAS No.:3800-06-4
- LM 22A4
Catalog No.:BCC6239
CAS No.:37988-18-4
- Geranylacetone
Catalog No.:BCN7567
CAS No.:3796-70-1
- tenofovir diphosphate
Catalog No.:BCC6447
CAS No.:166403-66-3
- Tenofovir Alafenamide Fumarate
Catalog No.:BCC8067
CAS No.:379270-38-9
- Tenofovir alafenamide
Catalog No.:BCC8066
CAS No.:379270-37-8
- Saracatinib (AZD0530)
Catalog No.:BCC1166
CAS No.:379231-04-6
- Cimifugin
Catalog No.:BCN5433
CAS No.:37921-38-3
- Jolkinolide B
Catalog No.:BCN2391
CAS No.:37905-08-1
- Jolkinolide A
Catalog No.:BCN3771
CAS No.:37905-07-0
- NBMPR
Catalog No.:BCC7516
CAS No.:38048-32-7
- alpha-Epoxydihydroartemisinic acid
Catalog No.:BCN5434
CAS No.:380487-65-0
- 187-1, N-WASP inhibitor
Catalog No.:BCC5866
CAS No.:380488-27-7
- DQP 1105
Catalog No.:BCC6205
CAS No.:380560-89-4
- CMPDA
Catalog No.:BCC6151
CAS No.:380607-77-2
- [(pF)Phe4]Nociceptin(1-13)NH2
Catalog No.:BCC5778
CAS No.:380620-88-2
- Pterolactam
Catalog No.:BCN5435
CAS No.:38072-88-7
- Bosutinib (SKI-606)
Catalog No.:BCC1167
CAS No.:380843-75-4
- TACA
Catalog No.:BCC6564
CAS No.:38090-53-8
- Perampanel
Catalog No.:BCC1847
CAS No.:380917-97-5
- Streptomycin sulfate
Catalog No.:BCC4851
CAS No.:3810-74-0
- Brevianamide F
Catalog No.:BCN6452
CAS No.:38136-70-8
Anti-inflammatory effect of coumarins isolated from Corydalis heterocarpa in HT-29 human colon carcinoma cells.[Pubmed:19500635]
Food Chem Toxicol. 2009 Aug;47(8):2129-34.
We investigated anti-inflammatory effects of two coumarins, columbianetin (A) and libanoridin (B), isolated from Corydalis heterocarpa in lipopolysaccharide (LPS)-stimulated HT-29 human colon carcinoma cells. Treatment with compound B inhibited the protein expression levels of inflammatory mediators such as inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), tumor necrosis factor-alpha (TNF-alpha), and interleukin-1 beta (IL-1 beta) in a dose-dependent manner in LPS-stimulated HT-29 cells, but compound A did not. Also, compound B had a higher inhibitory effect on production of cytokines such as IL-1 beta and TNF-alpha in LPS-stimulated HT-29 human colon carcinoma cells than those of compound A. Furthermore, we confirmed that LPS-induced transcription activity of NF-kappaB was inhibited by compound B. As a result of this study, compound B can be considered as a potential anti-inflammatory agent.
The pharmacokinetics and oral bioavailability studies of columbianetin in rats after oral and intravenous administration.[Pubmed:23994338]
J Ethnopharmacol. 2013 Oct 28;150(1):175-80.
ETHNOPHARMACOLOGICAL RELEVANCE: The roots of Angelica pubescens Maxim. f. biserrata Shan et Yuan (RAP) has been used as Traditional Chinese medicine to treat rheumatic disease in China since ancient times, but its action mechanisms was not well understood. Columbianetin is one of the main active constituents isolated from RAP, which has been shown to have various biological activities, but the absorption characteristics and oral bioavailability dose proportionality of columbianetin in vivo were not studied. MATERIALS AND METHODS: Male Sprague Dawley rats (210-230 g) received either an intravenous (i.v. 5, 10 and 20 mg kg(-1)) or oral (5, 10 and 20 mg kg(-1)) dose of columbianetin. The levels of columbianetin in plasma were measured by a simple and sensitive reversed-phase high-performance liquid chromatography (HPLC) method. The simple liquid-liquid extraction with ethyl acetate was used for sample preparation. Osthole was selected as internal standard (IS). RESULTS: The chromatographic separation was accomplished on a C18 column at a flow rate of 1 mL min(-1), where water-methanol was used as mobile phase. The calibration curve of the method was linear in the concentration range of 0.05-2000 mug mL(-1). The intra and inter-day accuracy for columbianetin in rat plasma samples were within 8% and the variation was less than 8.3%. This method was suitable for the determination and pharmacokinetic study of columbianetin in rat plasma after both intravenous and oral administration. The results indicated that maximum plasma concentrations(Cmax) for the columbianetin (17-42 mug mL(-1)) were achieved at 0.3-0.5h post-oral dosing and the apparent volume of distribution (V/F) ranged from 0.38 to 0.44 L. Absolute bioavailability of columbianetin was assessed to be 81.13 +/- 45.85, 81.09 +/- 33.63 and 54.30 +/- 23.19%, respectively. Terminal elimination half-life (T1/2) of the columbianetin after oral dosing was 60-90 min and were 2.5-3.3 fold longer than those observed for the i.v. dosing. CONCLUSIONS: The pharmacokinetic properties of columbianetin in rat after oral administration were characterized as rapid oral absorption, quick clearance and good absolute bioavailability. The bioavailability of columbianetin ranged from 54 to 81% for 5, 10 and 20 mg kg(-1) oral doses. The bioavailability of columbianetin is independent of the doses studied. Columbianetin showed dose proportionality over the dose range 5-20 mg kg(-1). The results clearly demonstrated that columbianetin was one of the material bases of RAP. Furthermore, an HPLC method was demonstrated in this study for the research of traditional Chinese medicine.
Anti-inflammatory effect of Columbianetin on activated human mast cells.[Pubmed:19483309]
Biol Pharm Bull. 2009 Jun;32(6):1027-31.
In the present study, we extracted Corydalis heterocarpa with various solvents in order to find the bioactive constituents that demonstrated anti-inflammatory effects. We isolated the active compound, Columbianetin. Anti-inflammatory effect of Columbianetin has been reported but the precise effects of Columbianetin in experimental models have remained unknown. In the present study, we investigate the effect of Columbianetin on the production of histamine, interleukin (IL)-1beta, IL-6, IL-8, and tumor necrosis factor (TNF)-alpha and expression of cyclooxygenase-2 (COX-2) by using the human mast cell line (HMC-1). Various concentrations of Columbianetin were treated before the activation of HMC-1 cells with phorbol 12-myristate 13-acetate (PMA) plus calcium ionophore, A23187. PMA plus A23187 significantly increased IL-1beta, IL-6, IL-8, and TNF-alpha production compared with media control (p<0.05). We also show that the increased cytokines IL-1beta, IL-6, IL-8, and TNF-alpha level was significantly inhibited by Columbianetin in a dose-dependent manner (p<0.05). Maximal inhibition rates of IL-1beta, IL-6, IL-8, and TNF-alpha production by Columbianetin were about 102.6%, 101.1%, 95.8%, and 103.9%, respectively. Columbianetin inhibited expression of COX-2. In addition, the effect of Columbianetin was investigated on the histamine release from HMC-1 stimulated by substance P, which promotes histamine release. Columbianetin also inhibited the histamine release by substance P. In conclusion, these results indicate that Columbianetin may be helpful in regulating mast cell-mediated allergic inflammatory responses.
Protective effect of (2'S)-columbianetin from Corydalis heterocarpa on UVB-induced keratinocyte damage.[Pubmed:22321694]
J Photochem Photobiol B. 2012 Apr 2;109:20-7.
A salt tolerant plant, Corydalis heterocarpa has been used as a folk medicine to treat travail and spasm. Recent studies have also reported antioxidant and antiinflammatory activities of compounds isolated from C. heterocarpa. In this study, the protective effect of (2'S)-columbianetin isolated from C. heterocarpa on UVB-induced human keratinocyte (HaCaT) damage was investigated. First, the appropriate energy level of UVB irradiation was determined using MTT and LDH assays. And then the protective effect of (2'S)-columbianetin on UVB induced HaCaT damage was evaluated by measuring; the changes in cell viability, LDH release level, ROS generation, cell cycle arrest and MMP expression levels. Finally, the effect of compound on MAPK and AP-1 signaling pathways were studied to understand the underlying signaling mechanisms. Result demonstrated that the presence of (2'S)-columbianetin suppressed the reactive oxygen species (ROS) generation, cell cycle arrest at sub-G1 phase and down regulation of MMP expression in UVB treated HaCaT cells. Furthermore, stress activated signaling pathways (ASK1-MAPK) and AP-1 signaling pathway were regulated by (2'S)-columbianetin treatment. These results suggest that (2'S)-columbianetin could be effectively used to protect human keratinocytes from UVB induced damage.
Tissue distribution study of columbianadin and its active metabolite columbianetin in rats.[Pubmed:26115176]
Biomed Chromatogr. 2016 Feb;30(2):256-62.
Columbianadin, one of the main bioactive constituents of the roots of Angelica pubescens Maxim. f. biserrata Shan et Yuan, has been found to possess obvious pharmacological effects in previous studies. In this study, a valid and sensitive reverse-phase high-performance liquid chromatography (RP-HPLC) method was established and validated for the determination of columbianadin (CBN) and its active metabolite columbianetin (CBT) in rat tissue samples. Sample separation was performed on an RP-HPLC column using a mobile phase of MeOH-H2 O (75:25, v/v) at a flow rate of 1.0 mL/min. The UV absorbance of the samples was measured at the wavelength 325 nm. The calibration curves for CBN were linear over the ranges of 0.5-20 microg/g for brain, testes and muscle, 1.0-10.0 microg/g for stomach and intestine, and 0.2-20.0 microg/g for heart, liver, spleen, lung and kidney. The calibration curves for CBT were linear over the ranges of 0.5-25 microg/g for stomach and intestine, and 0.1-10.0 microg/g for heart, liver, spleen, lung and kidney. The analysis method was successfully applied to a tissue distribution study of CBN and CBT after intravenous administration of CBN to rats. The results of this study indicated that CBN could be detected in all of the selected tissues after i.v. administration. CBN was distributed to rat tissues rapidly and could be metabolized to CBT in most detected tissues. Of the detected tissues, heart had the highest uptake of CBN, which suggested that heart might be one of the main target tissues of CBN. Concentrations of CBT were obviously higher in the digestive system than in other assayed tissues. The information provided by this research is very useful for gaining knowledge of the capacities of CBN and CBT to access different tissues.


