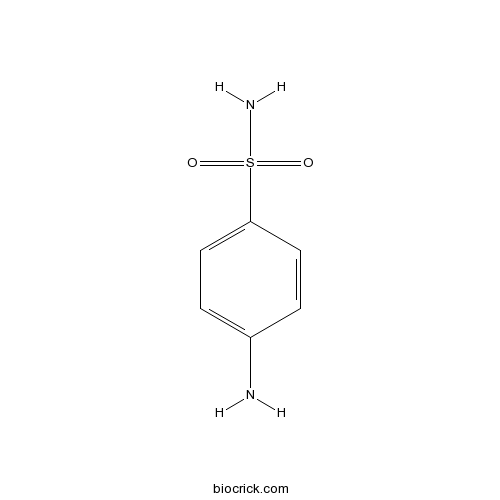
SulfanilamideCAS# 63-74-1 |
- Rocilinostat (ACY-1215)
Catalog No.:BCC2144
CAS No.:1316214-52-4
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
- Doxorubicin
Catalog No.:BCC2082
CAS No.:23214-92-8
- E 64d
Catalog No.:BCC1127
CAS No.:88321-09-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 63-74-1 | SDF | Download SDF |
| PubChem ID | 5333 | Appearance | Powder |
| Formula | C6H8N2O2S | M.Wt | 172.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Sulphanilamide | ||
| Solubility | DMSO : 100 mg/mL (580.72 mM; Need ultrasonic) H2O : 10 mg/mL (58.07 mM; Need ultrasonic) | ||
| Chemical Name | 4-aminobenzenesulfonamide | ||
| SMILES | Nc1ccc(cc1)[S](N)(=O)=O | ||
| Standard InChIKey | FDDDEECHVMSUSB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H8N2O2S/c7-5-1-3-6(4-2-5)11(8,9)10/h1-4H,7H2,(H2,8,9,10) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sulfanilamide is a competitive inhibitor for bacterial enzyme dihydropteroate synthetase with IC50 of 320 μM.
Target: dihydropteroate synthetase; Antibacterial
Sulfanilamide containing the sulfonamide functional group displays inhibitory activity for dihydropteroate synthetase partially purified from Escherichia coli which normally uses para-aminobenzoic acid (PABA) for synthesizing the necessary folic acid acting as a coenzyme in the synthesis of purine, pyrimidine and other amino acids, exhibiting an IC 50 of 320 μM for dihydropteroate synthetasea and Km of 2.5 uM for PABA [1]. Sulfanilamide shows IC50 of 286.8 μg/mL for recombinant S. cerevisiae strains with wild-type FOL1 genes, but the single mutation 55Trp to 55Ala or 57Pro to 57Ser within the putative active site of the fungal DHPS confers resistance to Sulfanilamide with IC50 of >800 μg/mL [2]. Administration of Sulfanilamide with the dosage of 100 mg/kg/day is effective in the prevention of P. carinii infection in the immunosuppressed rat model. When the dosage of sulfaguanidine and Sulfanilamide reduced to 10 mg/kg/day, breakthrough P. carinii infection occurs in the rats [3]. References: | |||||

Sulfanilamide Dilution Calculator

Sulfanilamide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.8072 mL | 29.036 mL | 58.072 mL | 116.144 mL | 145.18 mL |
| 5 mM | 1.1614 mL | 5.8072 mL | 11.6144 mL | 23.2288 mL | 29.036 mL |
| 10 mM | 0.5807 mL | 2.9036 mL | 5.8072 mL | 11.6144 mL | 14.518 mL |
| 50 mM | 0.1161 mL | 0.5807 mL | 1.1614 mL | 2.3229 mL | 2.9036 mL |
| 100 mM | 0.0581 mL | 0.2904 mL | 0.5807 mL | 1.1614 mL | 1.4518 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Sulfanilamide is a competitive inhibitor for bacterial enzyme dihydropteroate synthetase with IC50 of 320 μM.
- H-Met-OH
Catalog No.:BCC2993
CAS No.:63-68-3
- Primaquine Diphosphate
Catalog No.:BCC4706
CAS No.:63-45-6
- Androstenedione
Catalog No.:BCC8296
CAS No.:63-05-8
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
- 6-Aminoquinoxaline
Catalog No.:BCC8767
CAS No.:6298-37-9
- XL335
Catalog No.:BCC4501
CAS No.:629664-81-9
- Boc-Tle-OH
Catalog No.:BCC3343
CAS No.:62965-35-9
- Gnetucleistol D
Catalog No.:BCN3400
CAS No.:629643-26-1
- Gomisin G
Catalog No.:BCN2269
CAS No.:62956-48-3
- Gomisin F
Catalog No.:BCN3625
CAS No.:62956-47-2
- (2-Benzothiazolylthio)acetic acid
Catalog No.:BCC8387
CAS No.:6295-57-4
- 6-Methoxy-4-methylcoumarin
Catalog No.:BCN6537
CAS No.:6295-35-8
- L-Phenylalanine
Catalog No.:BCN3818
CAS No.:63-91-2
- Phenoxybenzamine HCl
Catalog No.:BCC4334
CAS No.:63-92-3
- Nonacosane
Catalog No.:BCC9102
CAS No.:630-03-5
- Ouabain
Catalog No.:BCC5069
CAS No.:630-60-4
- Phenytoin sodium
Catalog No.:BCC5071
CAS No.:630-93-3
- Corynoxeine
Catalog No.:BCN5002
CAS No.:630-94-4
- Neoprzewaquinone A
Catalog No.:BCN4169
CAS No.:630057-39-5
- MRS 2500 tetraammonium salt
Catalog No.:BCC5881
CAS No.:630103-23-0
- PD 168077 maleate
Catalog No.:BCC6919
CAS No.:630117-19-0
- AST 487
Catalog No.:BCC1373
CAS No.:630124-46-8
- Androstenone hydrazone
Catalog No.:BCC8830
CAS No.:63015-10-1
- Crenulatin
Catalog No.:BCN7791
CAS No.:63026-02-8
Pharmacokinetics of the antimicrobial drug Sulfanilamide is altered in a preclinical model of vascular calcification.[Pubmed:28042884]
Clin Exp Pharmacol Physiol. 2017 Dec;44 Suppl 1:99-106.
In vascular smooth muscle, calcium overload is linked to advancing age. The pharmacokinetics of Sulfanilamide (SA), a compound with antibacterial properties, was evaluated in a preclinical model of vascular calcification. SA was used since it is useful to study possible modifications in the renal and hepatic management of drugs. Vascular calcification was induced by administration of a single high dose of vitamin D3 to rats (treated group) 10 days before the experiments. A parallel control group was processed. The decrease of renal blood flow due to calcification of the renal arteries explains, at least in part, the decrease in the renal clearance of SA observed in treated rats. The liver metabolic function increased in treated rats as demonstrated by increases in plasma appearance rate of acetylated-Sulfanilamide (ASA), hepatic ASA content and hepatic N-acetyltransferase activity. The decrease in renal excretion of SA was not completely compensated by the hepatic metabolism increase, since the elimination rate of SA from the central compartment (K1-0 ) decreased in the treated group. In summary, in this experimental model with sustained arterial calcinosis induced by a single high dose of vitamin D3 10 days before the experiments, the pharmacokinetics of an aminobenzenesulfonamide is modified, at least in part, by the increase in the activity of hepatic N-acetyltransferase and the decrease in renal blood flow. This study emphasizes the importance of considering the presence of vascular calcification when a drug dose scheme is performed, in order to optimize pharmacotherapeutic results.
Peculiar behavior of starch 2,3-dialdehyde towards sulfanilamide and sulfathiazole.[Pubmed:27516312]
Carbohydr Polym. 2016 Nov 5;152:624-631.
In this study, starch (1) was oxidized to starch-2,3-dialdehyde (DAS; 2) using potassium periodate. In addition, two novel Schiff's bases (5 &6) were synthesized via a condensation reaction between DAS (2) and sulfa drugs (Sulfanilamide; 3 & sulfathiazole; 4). The synthesized Schiff's bases (5 &6) were characterized by FT-IR spectroscopy, X-ray diffraction and DSC analysis. DAS can easily be oxidized owing to its high aldehyde content (91.0%). However, it has low reactivity towards Sulfanilamide (3) and sulfathiazole (4). According to the diffraction functional theory, this peculiar behavior is caused by the absence of V-shape in alpha-glucan linkage in DAS molecules, making the carbonyl group least electropositive. This reduces the nucleophilic attacks of the amino group in sulfa drugs towards the carbonyl group in DAS.
Automated Enrichment of Sulfanilamide in Milk Matrices by Utilization of Aptamer-Linked Magnetic Particles.[Pubmed:27933990]
J Agric Food Chem. 2016 Dec 7;64(48):9246-9252.
The present work demonstrates the first automated enrichment approach for antibiotics in milk using specific DNA aptamers. First, aptamers toward the antibiotic Sulfanilamide were selected and characterized regarding their dissociation constants and specificity toward relevant antibiotics via fluorescence assay and LC-MS/MS detection. The performed enrichment was automated using the KingFisherDuo and compared to a manual approach. Verifying the functionality, trapping was realized in different milk matrices: (i) 0.3% fat milk, (ii) 1.5% fat milk, (iii) 3.5% fat milk, and (iv) 0.3% fat cocoa milk drink. Enrichment factors up to 8-fold could be achieved. Furthermore, it could be shown that novel implementation of a magnetic separator increases the reproducibility and reduces the hands-on time from approximately half a day to 30 min.
Ag loaded WO3 nanoplates for efficient photocatalytic degradation of sulfanilamide and their bactericidal effect under visible light irradiation.[Pubmed:27450332]
J Hazard Mater. 2016 Nov 15;318:407-416.
Sulfonamides (SAs) are extensively used antibiotics and their residues in the water bodies propose potential threat to the public. In this study, degradation efficiency of Sulfanilamide (SAM), which is the precursor of SAs, using WO3 nanoplates and their Ag heterogeneous as photocatalysts was investigated. WO3 nanoplates with uniform size were synthesized by a facile one step hydrothermal method. Different amount of Ag nanoparticles (Ag NPs) were loaded onto WO3 nanoplates using a photo-reduction method to generate WO3/Ag composites. The physio-chemical properties of synthesized nanomaterials were systematically characterized. Photodegradation of SAM by WO3 and WO3/Ag composites was conducted under visible light irradiation. The results show that WO3/Ag composites performed much better than pure WO3 where the highest removal rate was 96.2% in 5h. Ag as excellent antibacterial agent also endows certain antibacterial efficiency to WO3, and 100% removal efficiency against Escherichia Coli and Bacillus subtilis could be achieved in 2h under visible light irradiation for all three WO3/Ag composites synthesized. The improved performance in terms of SAM degradation and antibacterial activity of WO3/Ag can be attributed to the improved electron-hole pair separation rate where Ag NPs act as effective electron trapper during the photocatalytic process.


