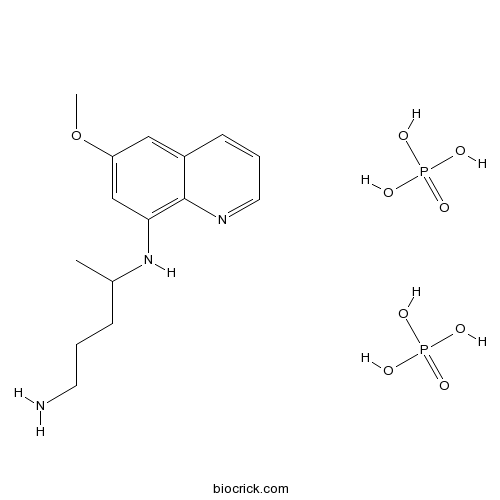
Primaquine Diphosphateantimalarial agent,blocks protein synthesis CAS# 63-45-6 |
- CP 31398 dihydrochloride
Catalog No.:BCC2406
CAS No.:1217195-61-3
- Tenovin-1
Catalog No.:BCC2239
CAS No.:380315-80-0
- PRIMA-1
Catalog No.:BCC2413
CAS No.:5608-24-2
- Pifithrin-α (PFTα)
Catalog No.:BCC2241
CAS No.:63208-82-2
- NSC 319726
Catalog No.:BCC2242
CAS No.:71555-25-4
- PhiKan 083
Catalog No.:BCC2411
CAS No.:880813-36-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 63-45-6 | SDF | Download SDF |
| PubChem ID | 6135 | Appearance | Powder |
| Formula | C15H27N3O9P2 | M.Wt | 455.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Primaquine phosphate; Primaquine bisphosphate | ||
| Solubility | H2O : 50 mg/mL (109.81 mM; Need ultrasonic) | ||
| Chemical Name | 4-N-(6-methoxyquinolin-8-yl)pentane-1,4-diamine;phosphoric acid | ||
| SMILES | CC(CCCN)NC1=C2C(=CC(=C1)OC)C=CC=N2.OP(=O)(O)O.OP(=O)(O)O | ||
| Standard InChIKey | GJOHLWZHWQUKAU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H21N3O.2H3O4P/c1-11(5-3-7-16)18-14-10-13(19-2)9-12-6-4-8-17-15(12)14;2*1-5(2,3)4/h4,6,8-11,18H,3,5,7,16H2,1-2H3;2*(H3,1,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Primaquine is the only generally available anti-malarial that prevents relapse in vivax and ovale malaria, and the only potent gametocytocide in falciparum malaria. References: | |||||

Primaquine Diphosphate Dilution Calculator

Primaquine Diphosphate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1962 mL | 10.9808 mL | 21.9616 mL | 43.9232 mL | 54.904 mL |
| 5 mM | 0.4392 mL | 2.1962 mL | 4.3923 mL | 8.7846 mL | 10.9808 mL |
| 10 mM | 0.2196 mL | 1.0981 mL | 2.1962 mL | 4.3923 mL | 5.4904 mL |
| 50 mM | 0.0439 mL | 0.2196 mL | 0.4392 mL | 0.8785 mL | 1.0981 mL |
| 100 mM | 0.022 mL | 0.1098 mL | 0.2196 mL | 0.4392 mL | 0.549 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: Not available.
Primaquine, an 8-aminoquinoline, is introduced as a curative antimalarial agent in 1950. Since then, the drug has been applied extensively to against the exoerythrocytic stage of malaria. It is demonstrated tthat primaquine, by binding to nucleic acids, could therefore block protein synthesis, alter lipid synthesis and interact with biological membranes. [1]
In vitro: Chicken embryo cells (CEC) model were adopted to investigate the effect of primaquine on Newcastle disease virus replication. It was found that Virus-induced hemadsorption was inhibited by primaquine in a dose-dependent manner and was completely suppressed by primaquine 250 g/ml. viral ribonucleic acid (RNA) synthesis was found to be suppressed when primaquine was added early in the virus replication cycle. Whereas, when the drug was added late in the cycle, RNA synthesis was stimulation. [1]
In vivo: Primaquine liposomes were labelled by 99mTc and injected intravenously to Swiss Albino mice. After injection, the major accumulation organ of liposomes was liver followed by spleen, pancreas, lungs and the others. Findings also suggested that Primaquine could block the eradication of the parasites and prevent relapse by destruction of the exoerythrocytic liver stages. [2]
Clinical trial: In a double-blind, randomized and placebo-controlled clinical trial, subjects were administered with chloroquine plus two primaquine diphosphate tablets (30 mg) daily or matching placebos in a two-to-one allocation. Chloroquine/primaquine treatment showed remarkable protective efficacy for a group of 100 subjects. Compared with that for the placebo treatment group of 51 subjects, chloroquine/primaquine exhibited inhibitory effect to 88% of all types malaria, 89% of P. falciparum malaria and 88% of P. vivax malaria. [3]
References:
[1]Burdick JR and Durand DP. Primaquine diphosphate: inhibition of newcastle disease virus replication. Antimicrob Agents Ch. 1974 Oct 15; 6(4): 460-4.
[2]Aricat B, Ozert AY, Ercans MT And Hincalt AA. Characterization, in vitro and in vivo studies on primaquine diphosphate liposomes. J. Microencapsulation. 1995; 12(5): 469-85.
[3]Soto J, Toledo J, Rodriquez M, Sanchez J, Herrera R, Padilla J and Berman J. Double-blind, randomized, placebo-controlled assessment of chloroquine/primaquine prophylaxis for malaria in nonimmune colombian soldiers. Clin Infect Dis. 1999 Jul; 29: 199-201.
- Androstenedione
Catalog No.:BCC8296
CAS No.:63-05-8
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
- 6-Aminoquinoxaline
Catalog No.:BCC8767
CAS No.:6298-37-9
- XL335
Catalog No.:BCC4501
CAS No.:629664-81-9
- Boc-Tle-OH
Catalog No.:BCC3343
CAS No.:62965-35-9
- Gnetucleistol D
Catalog No.:BCN3400
CAS No.:629643-26-1
- Gomisin G
Catalog No.:BCN2269
CAS No.:62956-48-3
- Gomisin F
Catalog No.:BCN3625
CAS No.:62956-47-2
- (2-Benzothiazolylthio)acetic acid
Catalog No.:BCC8387
CAS No.:6295-57-4
- 6-Methoxy-4-methylcoumarin
Catalog No.:BCN6537
CAS No.:6295-35-8
- Morusinol
Catalog No.:BCN4168
CAS No.:62949-93-3
- Mulberrin
Catalog No.:BCN4167
CAS No.:62949-79-5
- H-Met-OH
Catalog No.:BCC2993
CAS No.:63-68-3
- Sulfanilamide
Catalog No.:BCC4858
CAS No.:63-74-1
- L-Phenylalanine
Catalog No.:BCN3818
CAS No.:63-91-2
- Phenoxybenzamine HCl
Catalog No.:BCC4334
CAS No.:63-92-3
- Nonacosane
Catalog No.:BCC9102
CAS No.:630-03-5
- Ouabain
Catalog No.:BCC5069
CAS No.:630-60-4
- Phenytoin sodium
Catalog No.:BCC5071
CAS No.:630-93-3
- Corynoxeine
Catalog No.:BCN5002
CAS No.:630-94-4
- Neoprzewaquinone A
Catalog No.:BCN4169
CAS No.:630057-39-5
- MRS 2500 tetraammonium salt
Catalog No.:BCC5881
CAS No.:630103-23-0
- PD 168077 maleate
Catalog No.:BCC6919
CAS No.:630117-19-0
- AST 487
Catalog No.:BCC1373
CAS No.:630124-46-8
Analysis of quinocide in unprocessed primaquine diphosphate and primaquine diphosphate tablets using gas chromatography-mass spectrometry with supersonic molecular beams.[Pubmed:19108846]
J Chromatogr A. 2009 Jan 30;1216(5):824-9.
Malaria is one of the most widespread and deadly diseases on the planet. Every year, about 500 million new cases are diagnosed, and the annual death toll is about 3 million. Primaquine has strong antiparasitic effects against gametocytes and can therefore prevent the spread of the parasite from treated patients to mosquitoes. It is also used in radical cures and prevents relapse. Consequently, primaquine is an often-used drug. In this study the separation of unprocessed primaquine from the contaminant quinocide based on gas chromatography-mass spectrometry with supersonic molecular beam (SMB) is presented and 7.5 mg Primaquine Diphosphate tablets were analyzed. We present a novel method for fast determination of quinocide which is an isomer of primaquine as the main contaminant in unprocessed primaquine and in its medical form as tablets by gas chromatography-mass spectrometry with SMB (also named supersonic GC-MS). Supersonic GC-MS provides enhanced molecular ion without any ion source related peak tailing plus extended range of compounds amenable for GC-MS analysis. In addition, major isomer mass spectral effects were revealed in the mass spectra of primaquine and quinocide which facilitated the unambiguous identification of quinocide in primaquine tablets. Fast GC-MS analysis is demonstrated with less then 2 min elution time of the drug and its main contaminants.
Optimization of primaquine diphosphate tablet formulation for controlled drug release using the mixture experimental design.[Pubmed:22670808]
Pharm Dev Technol. 2013 Sep-Oct;18(5):1247-54.
A tablet formulation based on hydrophilic matrix with a controlled drug release was developed, and the effect of polymer concentrations on the release of Primaquine Diphosphate was evaluated. To achieve this purpose, a 20-run, four-factor with multiple constraints on the proportions of the components was employed to obtain tablet compositions. Drug release was determined by an in vitro dissolution study in phosphate buffer solution at pH 6.8. The polynomial fitted functions described the behavior of the mixture on simplex coordinate systems to study the effects of each factor (polymer) on tablet characteristics. Based on the response surface methodology, a tablet composition was optimized with the purpose of obtaining a Primaquine Diphosphate release closer to a zero order kinetic. This formulation released 85.22% of the drug for 8 h and its kinetic was studied regarding to Korsmeyer-Peppas model, (Adj-R(2) = 0.99295) which has confirmed that both diffusion and erosion were related to the mechanism of the drug release. The data from the optimized formulation were very close to the predictions from statistical analysis, demonstrating that mixture experimental design could be used to optimize Primaquine Diphosphate dissolution from hidroxypropylmethyl cellulose and polyethylene glycol matrix tablets.
Nature of the main contaminant in the drug primaquine diphosphate: SFC and SFC-MS methods of analysis.[Pubmed:17079107]
J Pharm Biomed Anal. 2007 Feb 19;43(3):937-44.
The drug Primaquine Diphosphate is used for causative treatment of malaria. Using HPLC-MS and GC-MS, this research group was previously able to show that the main contaminant of primaquine is the positional isomer quinocide [I. Brondz, D. Mantzilas, U. Klein, D. Ekeberg, E. Hvattum, M.N. Lebedeva, F.S. Mikhailitsyn, G.D. Soulimanov, J. Roe, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 800 (2004) 211-223; I. Brondz, U. Klein, D. Ekeberg, D. Mantzilas, E. Hvattum, H. Schultz, F. S. Mikhailitsyn, Asian J. Chem. 17 (2005) 1678-1688]. Primaquine and quinocide are highly toxic substances which can have a number of side effects upon use in medical treatment. A standard for quinocide is not typically commercially available. In the present work, supercritical fluid chromatography-mass spectrometry (SFC-MS) with two different columns was used to achieve a shorter analysis time for the separation between the positional isomers quinocide and primaquine in Primaquine Diphosphate and to elucidate additional information about differences in their MS fragmentation. Unlike using HPLC-MS, it was possible to achieve the differential fragmentation of positional isomers at branching points using the SFC-MS technique. The desired short analysis time was achieved using SFC equipped with a Discovery HS F5 column and the differential fragmentation of positional isomers during SFC-MS provides information on the differences in the structure of these substances. Using a Chiralpak AD-H chiral column, it was possible to resolve the enantiomers in primaquine and separate quinocide from those enantiomers.


