Stearic AcidCAS# 57-11-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 57-11-4 | SDF | Download SDF |
| PubChem ID | 5281 | Appearance | Powder |
| Formula | C18H36O2 | M.Wt | 284.5 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | DMSO : 125 mg/mL (439.40 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | octadecanoic acid | ||
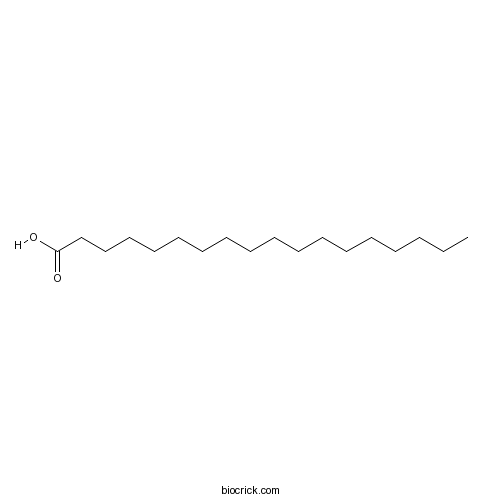
| SMILES | CCCCCCCCCCCCCCCCCC(=O)O | ||
| Standard InChIKey | QIQXTHQIDYTFRH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H36O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h2-17H2,1H3,(H,19,20) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Stearic Acid and its derivatives have been used as gelators in food and pharmaceutical gel formulations. Stearic Acid is a potent phosphatase 1B inhibitor, possibly causing an enhancement in the insulin receptor signaling to stimulate glucose uptake into adipocytes. Stearic Acid leads to dramatically reduced visceral fat likely by causing the apoptosis of preadipocytes, it also can reduce metastatic tumor burden. |
| Targets | Caspase | GLUT | Akt | MCP-1 |
| In vitro | Stearic acid serves as a potent inhibitor of protein tyrosine phosphatase 1B.[Pubmed: 24296476]Cell Physiol Biochem. 2013;32(5):1451-9.Free fatty acids (FFAs) are implicated in diverse signal transduction pathways. The present study investigated the effects of the saturated FFA Stearic Acid on protein tyrosine phosphatase 1B (PTP1B) activity, Akt activity, and glucose uptake into cells relevant to insulin signal. |
| In vivo | Dietary stearic acid leads to a reduction of visceral adipose tissue in athymic nude mice.[Pubmed: 25222131]PLoS One. 2014 Sep 15;9(9):e104083.Stearic Acid (C18:0) is a long chain dietary saturated fatty acid that has been shown to reduce metastatic tumor burden. |
| Animal Research | Milk production responses to dietary stearic acid vary by production level in dairy cattle.[Pubmed: 25529423]J Dairy Sci. 2015 Mar;98(3):1938-49.
|
| Structure Identification | Mater Sci Eng C Mater Biol Appl. 2015 Mar;48:688-99.Stearic acid based oleogels: a study on the molecular, thermal and mechanical properties.[Pubmed: 25579972 ]Stearic Acid and its derivatives have been used as gelators in food and pharmaceutical gel formulations. However, the mechanism pertaining to the Stearic Acid based gelation has not been deciphered yet. Keeping that in mind, we investigated the role of Stearic Acid on physic-chemical properties of oleogel. For this purpose, two different oil (sesame oil and soy bean oil) formulations/oleogels were prepared. |

Stearic Acid Dilution Calculator

Stearic Acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5149 mL | 17.5747 mL | 35.1494 mL | 70.2988 mL | 87.8735 mL |
| 5 mM | 0.703 mL | 3.5149 mL | 7.0299 mL | 14.0598 mL | 17.5747 mL |
| 10 mM | 0.3515 mL | 1.7575 mL | 3.5149 mL | 7.0299 mL | 8.7873 mL |
| 50 mM | 0.0703 mL | 0.3515 mL | 0.703 mL | 1.406 mL | 1.7575 mL |
| 100 mM | 0.0351 mL | 0.1757 mL | 0.3515 mL | 0.703 mL | 0.8787 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Palmitic acid
Catalog No.:BCN1206
CAS No.:57-10-3
- Flupirtine
Catalog No.:BCC4282
CAS No.:56995-20-1
- NSC 87877
Catalog No.:BCC2468
CAS No.:56990-57-9
- U 46619
Catalog No.:BCC7207
CAS No.:56985-40-1
- Boc-Cys(tBu)-OH
Catalog No.:BCC3379
CAS No.:56976-06-8
- Gabexate mesylate
Catalog No.:BCC2096
CAS No.:56974-61-9
- 9,9'-Di-O-(E)-feruloylsecoisolariciresinol
Catalog No.:BCN1415
CAS No.:56973-66-1
- Platyphyllenone
Catalog No.:BCN5766
CAS No.:56973-65-0
- Alnusdiol
Catalog No.:BCN6503
CAS No.:56973-51-4
- Withanolide B
Catalog No.:BCN8011
CAS No.:56973-41-2
- 2'-O-Galloylmyricitrin
Catalog No.:BCN8252
CAS No.:56939-52-7
- UBP 301
Catalog No.:BCC7172
CAS No.:569371-10-4
- Urea
Catalog No.:BCC8034
CAS No.:57-13-6
- Vincristine
Catalog No.:BCN5411
CAS No.:57-22-7
- Strychnine
Catalog No.:BCN4978
CAS No.:57-24-9
- Phenobarbital sodium salt
Catalog No.:BCC6230
CAS No.:57-30-7
- Pentobarbital sodium salt
Catalog No.:BCC6231
CAS No.:57-33-0
- Benactyzine hydrochloride
Catalog No.:BCC8841
CAS No.:57-37-4
- Phenytoin
Catalog No.:BCC5070
CAS No.:57-41-0
- Esromiotin
Catalog No.:BCC8325
CAS No.:57-47-6
- Fructose
Catalog No.:BCN4969
CAS No.:57-48-7
- Sucrose
Catalog No.:BCN5780
CAS No.:57-50-1
- Chlorotetracycline
Catalog No.:BCC8913
CAS No.:57-62-5
- Ethinyl Estradiol
Catalog No.:BCC3777
CAS No.:57-63-6
Dietary stearic acid leads to a reduction of visceral adipose tissue in athymic nude mice.[Pubmed:25222131]
PLoS One. 2014 Sep 15;9(9):e104083.
Stearic Acid (C18:0) is a long chain dietary saturated fatty acid that has been shown to reduce metastatic tumor burden. Based on preliminary observations and the growing evidence that visceral fat is related to metastasis and decreased survival, we hypothesized that dietary Stearic Acid may reduce visceral fat. Athymic nude mice, which are used in models of human breast cancer metastasis, were fed a Stearic Acid, linoleic acid (safflower oil), or oleic acid (corn oil) enriched diet or a low fat diet ad libitum. Total body weight did not differ significantly between dietary groups over the course of the experiment. However visceral fat was reduced by approximately 70% in the Stearic Acid fed group compared to other diets. In contrast total body fat was only slightly reduced in the Stearic Acid diet fed mice when measured by dual-energy x-ray absorptiometry and quantitative magnetic resonance. Lean body mass was increased in the Stearic Acid fed group compared to all other groups by dual-energy x-ray absorptiometry. Dietary Stearic Acid significantly reduced serum glucose compared to all other diets and increased monocyte chemotactic protein-1 (MCP-1) compared to the low fat control. The low fat control diet had increased serum leptin compared to all other diets. To investigate possible mechanisms whereby Stearic Acid reduced visceral fat we used 3T3L1 fibroblasts/preadipocytes. Stearic Acid had no direct effects on the process of differentiation or on the viability of mature adipocytes. However, unlike oleic acid and linoleic acid, Stearic Acid caused increased apoptosis (programmed cell death) and cytotoxicity in preadipocytes. The apoptosis was, at least in part, due to increased caspase-3 activity and was associated with decreased cellular inhibitor of apoptosis protein-2 (cIAP2) and increased Bax gene expression. In conclusion, dietary Stearic Acid leads to dramatically reduced visceral fat likely by causing the apoptosis of preadipocytes.
Stearic acid based oleogels: a study on the molecular, thermal and mechanical properties.[Pubmed:25579972]
Mater Sci Eng C Mater Biol Appl. 2015 Mar;48:688-99.
Stearic Acid and its derivatives have been used as gelators in food and pharmaceutical gel formulations. However, the mechanism pertaining to the Stearic Acid based gelation has not been deciphered yet. Keeping that in mind, we investigated the role of Stearic Acid on physic-chemical properties of oleogel. For this purpose, two different oil (sesame oil and soy bean oil) formulations/oleogels were prepared. In depth analysis of gel kinetics, gel microstructure, molecular interactions, thermal and mechanical behaviors of the oleogels were done. The properties of the oleogels were dependent on the type of the vegetable oil used and the concentration of the Stearic Acid. Avrami analysis of DSC thermograms indicated that heterogeneous nucleation was coupled with the one-dimensional growth of gelator fibers as the key phenomenon in the formation of oleogels. Viscoelastic and pseudoplastic nature of the oleogels was analyzed in-depth by fitting the stress relaxation data in modified Peleg's model and rheological studies, respectively. Textural studies have revealed that the coexistence of hydrogen bond dissipation and formation of new bonds is possible under stress conditions in the physical oleogels.
Milk production responses to dietary stearic acid vary by production level in dairy cattle.[Pubmed:25529423]
J Dairy Sci. 2015 Mar;98(3):1938-49.
Effects of Stearic Acid supplementation on feed intake and metabolic and production responses of dairy cows with a wide range of milk production (32.2 to 64.4 kg/d) were evaluated in a crossover design experiment with a covariate period. Thirty-two multiparous Holstein cows (142+/-55 d in milk) were assigned randomly within level of milk yield to treatment sequence. Treatments were diets supplemented (2% of diet dry matter) with Stearic Acid (SA; 98% C18:0) or control (soyhulls). The diets were based on corn silage and alfalfa and contained 24.5% forage neutral detergent fiber, 25.1% starch, and 17.3% crude protein. Treatment periods were 21 d with the final 4 d used for data and sample collection. Compared with the control, SA increased dry matter intake (DMI; 26.1 vs. 25.2 kg/d) and milk yield (40.2 vs. 38.5 kg/d). Stearic Acid had no effect on the concentration of milk components but increased yields of fat (1.42 vs. 1.35 kg/d), protein (1.19 vs. 1.14 kg/d), and lactose (1.96 vs. 1.87 kg/d). The SA treatment increased 3.5% fat-corrected milk (3.5% FCM; 40.5 vs. 38.6 kg/d) but did not affect feed efficiency (3.5% FCM/DMI, 1.55 vs. 1.53), body weight, or body condition score compared with the control. Linear interactions between treatment and level of milk yield during the covariate period were detected for DMI and yields of milk, fat, protein, lactose, and 3.5% FCM; responses to SA were positively related to milk yield of cows. The SA treatment increased crude protein digestibility (67.4 vs. 65.5%), tended to increase neutral detergent fiber digestibility (43.6 vs. 42.3%), decreased fatty acid (FA) digestibility (56.6 vs. 76.1%), and did not affect organic matter digestibility. Fatty acid yield response, calculated as the additional FA yield secreted in milk per unit of additional FA intake, was only 13.3% for total FA and 8.2% for C18:0 plus cis-9 C18:1. Low estimated digestibility of the SA supplement was at least partly responsible for the low FA yield response. Treatment did not affect plasma insulin, glucagon, glucose, and nonesterified FA concentrations. Results show that Stearic Acid has the potential to increase DMI and yields of milk and milk components, without affecting conversion of feed to milk, body condition score, or body weight. Moreover, effects on DMI and yields of milk and milk components were more pronounced for higher-yielding cows than for lower-yielding cows.
Stearic acid serves as a potent inhibitor of protein tyrosine phosphatase 1B.[Pubmed:24296476]
Cell Physiol Biochem. 2013;32(5):1451-9.
BACKGROUND/AIMS: Free fatty acids (FFAs) are implicated in diverse signal transduction pathways. The present study investigated the effects of the saturated FFA Stearic Acid on protein tyrosine phosphatase 1B (PTP1B) activity, Akt activity, and glucose uptake into cells relevant to insulin signal. METHODS: PTP1B activity was assayed under the cell-free conditions. Phosphorylation of insulin receptor and Akt and glucose uptake into cells were monitored in differentiated 3T3-L1-GLUT4myc adipocytes. RESULTS: In the cell-free PTP1B assay, Stearic Acid suppressed PTP1B activity in a concentration (1-30 muM)-dependent manner. For 3T3-L1- GLUT4myc adipocytes insulin phosphorylated insulin receptor at Tyr1185 and Akt at Thr308 and Ser473 in a concentration (100 fM-100 nM)-dependent manner and stimulated glucose uptake into cells in a concentration (0.1-100 nM)-dependent manner. Stearic Acid (30 muM) significantly increased insulin-induced phosphorylation of insulin receptor at Tyr1185, but insulin-induced phosphorylation of Akt was not significantly enhanced. Stearic Acid (30 muM) by itself promoted glucose uptake into adipocytes. CONCLUSION: The results of the present study indicate that Stearic Acid serves as a potent PTP1B inhibitor, possibly causing an enhancement in the insulin receptor signaling to stimulate glucose uptake into adipocytes.


