Benactyzine hydrochlorideCAS# 57-37-4 |
Quality Control & MSDS
Number of papers citing our products

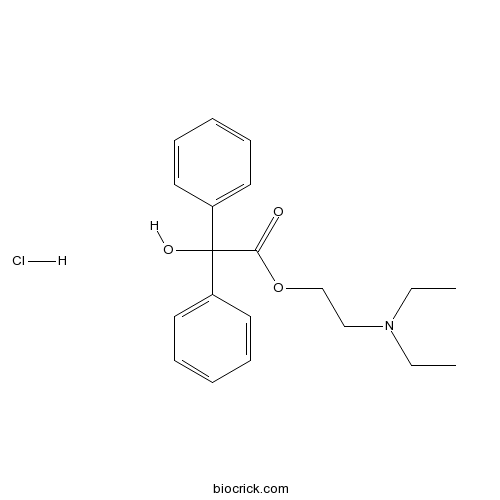
Chemical structure

3D structure
| Cas No. | 57-37-4 | SDF | Download SDF |
| PubChem ID | 66448 | Appearance | Powder |
| Formula | C20H26ClNO3 | M.Wt | 363.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 150 mg/mL (412.22 mM; Need ultrasonic and warming) | ||
| Chemical Name | 2-(diethylamino)ethyl 2-hydroxy-2,2-diphenylacetate;hydrochloride | ||
| SMILES | CCN(CC)CCOC(=O)C(C1=CC=CC=C1)(C2=CC=CC=C2)O.Cl | ||
| Standard InChIKey | ZCEHOOLYWQBGQO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H25NO3.ClH/c1-3-21(4-2)15-16-24-19(22)20(23,17-11-7-5-8-12-17)18-13-9-6-10-14-18;/h5-14,23H,3-4,15-16H2,1-2H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Benactyzine hydrochloride Dilution Calculator

Benactyzine hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.748 mL | 13.74 mL | 27.4801 mL | 54.9602 mL | 68.7002 mL |
| 5 mM | 0.5496 mL | 2.748 mL | 5.496 mL | 10.992 mL | 13.74 mL |
| 10 mM | 0.2748 mL | 1.374 mL | 2.748 mL | 5.496 mL | 6.87 mL |
| 50 mM | 0.055 mL | 0.2748 mL | 0.5496 mL | 1.0992 mL | 1.374 mL |
| 100 mM | 0.0275 mL | 0.1374 mL | 0.2748 mL | 0.5496 mL | 0.687 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Pentobarbital sodium salt
Catalog No.:BCC6231
CAS No.:57-33-0
- Phenobarbital sodium salt
Catalog No.:BCC6230
CAS No.:57-30-7
- Strychnine
Catalog No.:BCN4978
CAS No.:57-24-9
- Vincristine
Catalog No.:BCN5411
CAS No.:57-22-7
- Urea
Catalog No.:BCC8034
CAS No.:57-13-6
- Stearic Acid
Catalog No.:BCN3820
CAS No.:57-11-4
- Palmitic acid
Catalog No.:BCN1206
CAS No.:57-10-3
- Flupirtine
Catalog No.:BCC4282
CAS No.:56995-20-1
- NSC 87877
Catalog No.:BCC2468
CAS No.:56990-57-9
- U 46619
Catalog No.:BCC7207
CAS No.:56985-40-1
- Boc-Cys(tBu)-OH
Catalog No.:BCC3379
CAS No.:56976-06-8
- Gabexate mesylate
Catalog No.:BCC2096
CAS No.:56974-61-9
- Phenytoin
Catalog No.:BCC5070
CAS No.:57-41-0
- Esromiotin
Catalog No.:BCC8325
CAS No.:57-47-6
- Fructose
Catalog No.:BCN4969
CAS No.:57-48-7
- Sucrose
Catalog No.:BCN5780
CAS No.:57-50-1
- Chlorotetracycline
Catalog No.:BCC8913
CAS No.:57-62-5
- Ethinyl Estradiol
Catalog No.:BCC3777
CAS No.:57-63-6
- Probenecid
Catalog No.:BCC4832
CAS No.:57-66-9
- Sulfaguanidine
Catalog No.:BCC4727
CAS No.:57-67-0
- Sulfamethazine
Catalog No.:BCC4942
CAS No.:57-68-1
- Progesterone
Catalog No.:BCN2198
CAS No.:57-83-0
- Testosterone propionate
Catalog No.:BCC9172
CAS No.:57-85-2
- Ergosterol
Catalog No.:BCN5787
CAS No.:57-87-4
Effects of selected anticholinergics on acoustic startle response in rats.[Pubmed:11920928]
J Appl Toxicol. 2001 Dec;21 Suppl 1:S95-101.
The present study compared the effects of the anticholinergics aprophen hydrochloride, atropine sulfate, azaprophen hydrochloride, Benactyzine hydrochloride, biperiden hydrochloride, diazepam, procyclidine hydrochloride, scopolamine hydrobromide and trihexyphenidyl hydrochloride on acoustic startle response in rats. Peak startle amplitude, latency to peak startle amplitude and prepulse inhibition following 100- and 120-dB tones were recorded 15 min following drug administration in food-restricted rats. Aprophen, atropine, azaprophen, benactyzine, biperiden and scopolamine significantly increased peak startle amplitude and decreased latency to peak startle amplitude following 100-dB pulses. In contrast, only biperiden increased peak startle amplitude following 120-dB pulses, whereas atropine and trihexyphenidyl decreased latency to peak startle amplitude following 120-dB pulses. Benactyzine decreased prepulse inhibition following both 100- and 120-dB pulses, whereas both biperiden and scopolamine decreased prepulse inhibition following 120-dB pulses. Acoustic startle response measures were effective in differentiating the effects of anticholinergic compounds. The comparison of drug effects on the acoustic startle response may be useful in selecting efficacious anticholinergic drug therapies with a minimal range of side-effects. In addition, these data may be useful in down-selecting the number of anticholinergic drugs that need to be tested in comparison studies involving more complex behavioral tests.
Dose-response curves and time-course effects of selected anticholinergics on locomotor activity in rats.[Pubmed:10639682]
Psychopharmacology (Berl). 1999 Dec;147(3):250-6.
RATIONALE: In order to facilitate direct comparisons of anticholinergic drug effects on activity, nine drugs were tested in one laboratory using a standardized procedure. OBJECTIVE: The present study compared the effects of aprophen hydrochloride, atropine sulfate, azaprophen hydrochloride, Benactyzine hydrochloride, biperiden hydrochloride, diazepam, procyclidine hydrochloride, scopolamine hydrobromide, and trihexyphenidyl hydrochloride on activity levels in rats. METHODS: Both fine motor activity (reflecting smaller movements) and ambulatory activity (reflecting larger movements) were recorded for 23 h following drug administration in food-restricted rats. All drugs were administered during the light period of the photocycle. RESULTS: Atropine, azaprophen, biperiden, scopolamine, and trihexyphenidyl increased both ambulations and fine motor activity significantly during the first hour post-injection, but the increased activity levels returned to vehicle control levels within 2-6 h post-injection. Benactyzine and procyclidine only increased fine motor activity significantly above vehicle control levels and activity levels returned to vehicle control levels within 2-3 h. Finally, aprophen and diazepam generally did not increase measures of activity significantly above vehicle controls at the dose ranges examined. CONCLUSIONS: Based on potencies relative to scopolamine, the potency of the drugs could be ranked as follows: scopolamine > trihexyphenidyl > biperiden > azaprophen > procyclidine > benactyzine > atropine > aprophen. The comparison of drug effects on activity may be useful in selecting anticholinergic drug therapies with a minimal range of side effects. In addition, these data may reduce the number of anticholinergic drugs that need to be tested in comparison studies involving more complex behavioral tests.
An ethologically based, stimulus and gender-sensitive nonhuman primate model for anxiety.[Pubmed:8588065]
Prog Neuropsychopharmacol Biol Psychiatry. 1995 Jul;19(4):677-85.
1. Adult male and female squirrel monkeys were tested for behavioral responses to 5 min. social separation (alone in test room) followed by 30-sec. exposure to 2 humans wearing a leather capture glove. 2. Trials were preceded by intramuscular injection of an anticholinergic drug, Benactyzine hydrochloride, in doses of 0.0, 0.6, 1.0, 2.0, and 3.0 mg/kg. 3. Measured behaviors were number and type of vocalization and locomotor activity (duration in sec) in each of the two testing conditions. 4. A dose-response relationship for bark/yap vocalizations during the 30-sec trials was established, with 1.0 mg/kg being the most effective dose. 5. Males and females differed in the number of barks/yaps produced during 30-sec. trials at every drug dose. 6. The present testing paradigm provides the basis for efficiently determining the extent of gender differences in dose/response relationships for drugs of possible therapeutic value in the treatment of anxiety-related behavioral disorders.
Gender differences in reactivity of adult squirrel monkeys to short-term environmental challenges.[Pubmed:1686486]
Neurosci Biobehav Rev. 1991 Winter;15(4):469-71.
Evidence is presented to show that individual adult squirrel monkeys show gender-specific reactivity profiles to threatening stimuli under laboratory conditions, and that a putative anxiogenic drug, Benactyzine hydrochloride, enhances the vocal response to threatening stimuli, but otherwise preserves the relative importance of the stimuli to both males and females. These data support the conclusion that screening of putative anxiolytic drugs in a primate model can be accomplished using efficient, ethologically based testing procedures in the laboratory.
Conditioned taste aversion and cholinergic drugs: pharmacological antagonism.[Pubmed:3615551]
Pharmacol Biochem Behav. 1987 May;27(1):81-5.
The effectiveness of drugs as unconditioned stimuli (UCSs) in the conditioned taste aversion (CTA) procedure may be influenced by specific pharmacological antagonism. The present studies examined the UCS effects of two carbamates, physostigmine salicylate (PS) and pyridostigmine bromide (PB), and three anticholinergic compounds, atropine methyl nitrate (AMN), atropine sulfate (AS), and Benactyzine hydrochloride (BH). Individual drugs, as well as combinations of the carbamates and the anticholinergics, were studied in a two-bottle procedure in rats. The lowest effective doses for eliciting significant CTAs were PS, 0.32 mg/kg; PB, 1.00 mg/kg; AMN, 0.04 mg/kg; AS, 0.07 mg/kg and BH, 0.90 mg/kg, IP. Combinations of PS with either AMN or BH were mutually antagonistic as UCSs, whereas PS with AS was not. PB with AMN, but not with AS, also showed antagonism in the procedure. The present results suggest that the CTA procedure is well-suited for direct examination of cholinergic drug effects and may also be used to explore interactions of different classes of cholinergic drugs.
Effects of benactyzine hydrochloride on dynamic vision functions.[Pubmed:7150174]
Aviat Space Environ Med. 1982 Nov;53(11):1123-8.
We have investigated the effects of an anticholinergic drug, benactyzine HCl on vision and vision function. Our experiments assess the time course and severity of benactyzine effects on visual acuity for static and moving targets, amplitude and dynamics of accommodation, pupil response to light and the spatial contrast sensitivity function. A single dose of the drug (4.14 mg/70 kg body weight, which is within the therapeutic range) and placebo were administered intramuscularly to 12 subjects. Large and significant decrements in visual function were demonstrated after the administration of benactyzine, particularly for those functions performed at near focus. The drug effect was rapid in onset, beginning 7-10 min after injection, peaked at approximately 30-40 min, and declined to baseline values over the next 2 h. The drug increased pulse rate and blood pressure, and induced an intoxicated state involving loss of concentration, attention, and short-term memory.
Kinetics and stability of a multicomponent organophosphate antidote formulation in glass and plastic.[Pubmed:7069590]
J Pharm Sci. 1982 Mar;71(3):321-5.
An aqueous solution of trimedoxime bromide, atropine, and Benactyzine hydrochloride was formulated to have maximum stability as an antidote in organophosphorus poisoning. The stability of the mixture in glass and plastic cartridges was determined. Glass cartridges were more desirable than plastic; there was less vapor loss, color formation, and anomalous reaction. Trimedoxime was stable, losing 1.4% of its potency after 1 year at 25 degrees and atropine was more stable than trimedoxime. Considerable degradation of benactyzine occurred; 20% of its potency was lost after 1 year at 25 degrees. Equations for predicting the shelf life of each ingredient at selected temperatures are presented.
Ion-pair high-performance liquid chromatographic separation of a multicomponent anticholinergic drug formulation.[Pubmed:621264]
J Chromatogr. 1978 Feb 1;148(2):453-7.
N,N'-Trimethylene-bis-(pyridinium-4-aldoxime)dibromide, 4-pyridine aldoxime, atropine sulfate, Benactyzine hydrochloride, methyl paraben and propyl paraben are separated by ion-pair high-performance liquid chromatography. The method is specific for detecting and quantifying each compound in a complex mixture without solvent extraction or pretreatment. Levels as low as 1 ng on column are quantifiable by the procedure. All components are eluted within 9 min subsequent to the initial injection. Because of the simplicity of the method, the procedure is suitable for routinely monitoring the stability of the various compounds in the formulation during storage.


