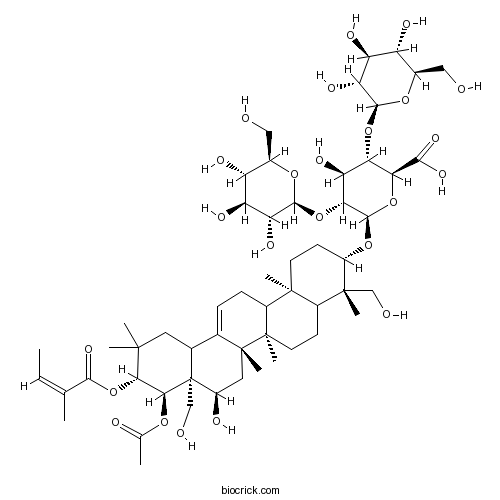
Sapogenins GlycosidesCAS# 8047-15-2 |
- CI994 (Tacedinaline)
Catalog No.:BCC2159
CAS No.:112522-64-2
- Tubastatin A HCl
Catalog No.:BCC3877
CAS No.:1310693-92-5
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- Mocetinostat (MGCD0103, MG0103)
Catalog No.:BCC2146
CAS No.:726169-73-9
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- AR-42 (OSU-HDAC42)
Catalog No.:BCC2161
CAS No.:935881-37-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 8047-15-2 | SDF | Download SDF |
| PubChem ID | 6540709 | Appearance | Powder |
| Formula | C55H86O24 | M.Wt | 1131.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : 33.33 mg/mL (Need ultrasonic) | ||
| Chemical Name | (2S,3S,4S,5R,6R)-6-[[(3S,4S,6aR,6bS,8R,8aR,9R,10R,14bR)-9-acetyloxy-8-hydroxy-4,8a-bis(hydroxymethyl)-4,6a,6b,11,11,14b-hexamethyl-10-[(Z)-2-methylbut-2-enoyl]oxy-1,2,3,4a,5,6,7,8,9,10,12,12a,14,14a-tetradecahydropicen-3-yl]oxy]-4-hydroxy-3,5-bis[[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy]oxane-2-carboxylic acid | ||
| SMILES | CC=C(C)C(=O)OC1C(C2(C(CC1(C)C)C3=CCC4C5(CCC(C(C5CCC4(C3(CC2O)C)C)(C)CO)OC6C(C(C(C(O6)C(=O)O)OC7C(C(C(C(O7)CO)O)O)O)O)OC8C(C(C(C(O8)CO)O)O)O)C)CO)OC(=O)C | ||
| Standard InChIKey | AXNVHPCVMSNXNP-ZELRDNAQSA-N | ||
| Standard InChI | InChI=1S/C55H86O24/c1-10-23(2)46(71)79-43-44(72-24(3)60)55(22-59)26(17-50(43,4)5)25-11-12-30-51(6)15-14-32(52(7,21-58)29(51)13-16-53(30,8)54(25,9)18-31(55)61)75-49-41(77-48-38(67)36(65)34(63)28(20-57)74-48)39(68)40(42(78-49)45(69)70)76-47-37(66)35(64)33(62)27(19-56)73-47/h10-11,26-44,47-49,56-59,61-68H,12-22H2,1-9H3,(H,69,70)/b23-10-/t26?,27-,28-,29?,30?,31-,32+,33-,34-,35+,36+,37-,38-,39+,40+,41-,42+,43+,44+,47+,48+,49-,51+,52-,53-,54-,55+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Sapogenins Glycosides Dilution Calculator

Sapogenins Glycosides Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.8839 mL | 4.4197 mL | 8.8394 mL | 17.6788 mL | 22.0985 mL |
| 5 mM | 0.1768 mL | 0.8839 mL | 1.7679 mL | 3.5358 mL | 4.4197 mL |
| 10 mM | 0.0884 mL | 0.442 mL | 0.8839 mL | 1.7679 mL | 2.2098 mL |
| 50 mM | 0.0177 mL | 0.0884 mL | 0.1768 mL | 0.3536 mL | 0.442 mL |
| 100 mM | 0.0088 mL | 0.0442 mL | 0.0884 mL | 0.1768 mL | 0.221 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Paeoniflorigenone
Catalog No.:BCN3933
CAS No.:80454-42-8
- Padmatin
Catalog No.:BCN4340
CAS No.:80453-44-7
- Dynorphin A
Catalog No.:BCC7596
CAS No.:80448-90-4
- gamma-Diasarone
Catalog No.:BCN4339
CAS No.:80434-33-9
- Calcium Levofolinate
Catalog No.:BCC4643
CAS No.:80433-71-2
- Notoginsenoside R2
Catalog No.:BCN3328
CAS No.:80418-25-3
- Notoginsenoside R1
Catalog No.:BCN1097
CAS No.:80418-24-2
- 13-O-p-Coumaroylplumieride
Catalog No.:BCN4338
CAS No.:80416-52-0
- Scorpioidine
Catalog No.:BCN2027
CAS No.:80405-18-1
- 7-Acetylscorpioidine
Catalog No.:BCN2028
CAS No.:80405-17-0
- Protoplumericin A
Catalog No.:BCN4572
CAS No.:80396-57-2
- Z-D-Thr-OH
Catalog No.:BCC2736
CAS No.:80384-27-6
- Fluticasone propionate
Catalog No.:BCC4907
CAS No.:80474-14-2
- Rubifolic acid
Catalog No.:BCN4341
CAS No.:80489-65-2
- Mezlocillin Sodium Monohydrate
Catalog No.:BCC5634
CAS No.:80495-46-1
- Tenacigenin B
Catalog No.:BCN4342
CAS No.:80508-42-5
- Glaucocalyxin B
Catalog No.:BCN8440
CAS No.:80508-81-2
- Veratrine
Catalog No.:BCN8444
CAS No.:8051-02-3
- Euchrestaflavanone A
Catalog No.:BCN3576
CAS No.:80510-05-0
- Grossamide
Catalog No.:BCN4571
CAS No.:80510-06-1
- Cis-N-Feruloyltyramine
Catalog No.:BCN3729
CAS No.:80510-09-4
- 7beta-(3-Ethyl-cis-crotonoyloxy)-1alpha-(2-methylbutyryloxy)-3,14-dehydro-Z-notonipetranone
Catalog No.:BCN7638
CAS No.:80514-14-3
- PHTPP
Catalog No.:BCC7447
CAS No.:805239-56-9
- Vitexin 2''-O-(4'''-O-acetyl)rhamnoside
Catalog No.:BCN6740
CAS No.:80537-98-0
Steroidal sapogenins and glycosides from the fibrous roots of Polygonatum odoratum with inhibitory effect on tissue factor (TF) procoagulant activity.[Pubmed:25042471]
Steroids. 2014 Nov;89:1-10.
Six new spirostane glycosides (1-6), named polygodosides A-F, one new furostanol glycoside, polygodoside G (7), one new cholestane glycoside, polygodoside H (8), and one new steroidal sapogenin, polygodosin A (9), together with thirteen known compounds (10-22) were isolated from a 90% MeOH extract of the fibrous roots of Polygonatum odoratum (Mill.) Druce. The structures of new compounds were elucidated by extensive 1D and 2D NMR spectroscopic analyses and mass spectrometry. The effects on TF procoagulant activity in THP-1 cells were tested for most of the compounds.
Steroidal sapogenins and glycosides from the rhizomes of Dioscorea bulbifera.[Pubmed:19842682]
J Nat Prod. 2009 Nov;72(11):1964-8.
Four new steroidal sapogenins (1-4), named diosbulbisins A-D, two new spirostane glycosides, diosbulbisides A (5) and B (6), one new cholestane glycoside, diosbulbiside C (7), and the known compounds 8-10 were isolated from rhizomes of Dioscorea bulbifera. Their structures were elucidated by 1D and 2D NMR techniques, HRFTMS, and chemical methods. The unusual furospirostanol sapogenin skeletons, as found in compounds 3 and 4, are reported in the family Dioscoreaceae for the first time. Cytotoxicity of compounds 1-10 was evaluated using two human hepatocellular carcinoma cell lines (Bel-7402 and SMMC7721).
Steroidal sapogenins and glycosides from the fibrous roots of Ophiopogon japonicus and Liriope spicata var. prolifera with anti-inflammatory activity.[Pubmed:25757489]
Chem Pharm Bull (Tokyo). 2015;63(3):187-94.
Two new steroidal glycosides (1 and 2), together with 15 known compounds (3-17) were isolated from the fibrous roots of Ophiopogon japonicus, and three new steroidal glycosides (18-20), together with 14 known compounds (21-34) were isolated from the fibrous roots of Liriope spicata var. prolifera. The structures of the new compounds were elucidated on the basis of extensive one-dimensional (1D)- and 2D-NMR spectroscopic analyses and mass spectrometry. The isolated compounds were evaluated for their anti-inflammatory activity in vitro. Most of these steroidal glycosides showed significant inhibitory activity against neutrophil respiratory burst stimulated by phorbol myristate acetate.
Isolation of solasodine and other steroidal alkaloids and sapogenins by direct hydrolysis-extraction of Solanum plants or glycosides therefrom.[Pubmed:11557084]
Phytochemistry. 2001 Oct;58(3):501-8.
Concomitant extraction and hydrolysis of Solanum steroidal glycoalkaloids in a two-phase system containing an aqueous mineral acid and an organic water immiscible solvent, and having a boiling point under 100(o)C, is described. It is essentially a "one-pot" process, combining direct acid hydrolysis of the glycosides in the plant material and in situ extraction of the released aglycones after alkali treatment, in a single step, the various ingredients being added simultaneously or sequentially, as required. Application of the process to fruits, leaves, and tissue cultures of Solanum khasianum Clarke plants, either fresh or in dried, finely ground form, using a two-phase aqueous hydrochloric acid-toluene system, proved to be very suitable for continuous production of pure solasodine which is a valuable raw steroid for the preparation of steroidal drugs. Pure Solanum glycoalkaloids were also prepared and hydrolysed accordingly. The process has analytical applications too, and could be extended in a general way to glycosides in other series for preparation of their related aglycones.


