SB505124ALK4/ALK5/ALK7 inhibitor CAS# 694433-59-5 |
- AS 19
Catalog No.:BCC7218
CAS No.:1000578-26-6
- Almotriptan Malate
Catalog No.:BCC5045
CAS No.:181183-52-8
- SB271046
Catalog No.:BCC5057
CAS No.:209481-20-9
- Lurasidone HCl
Catalog No.:BCC4458
CAS No.:367514-88-3
- Vortioxetine
Catalog No.:BCC2046
CAS No.:508233-74-7
- Urapidil HCl
Catalog No.:BCC5044
CAS No.:64887-14-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 694433-59-5 | SDF | Download SDF |
| PubChem ID | 9858940 | Appearance | Powder |
| Formula | C20H21N3O2 | M.Wt | 335.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 113.33 mg/mL (337.90 mM; Need ultrasonic) | ||
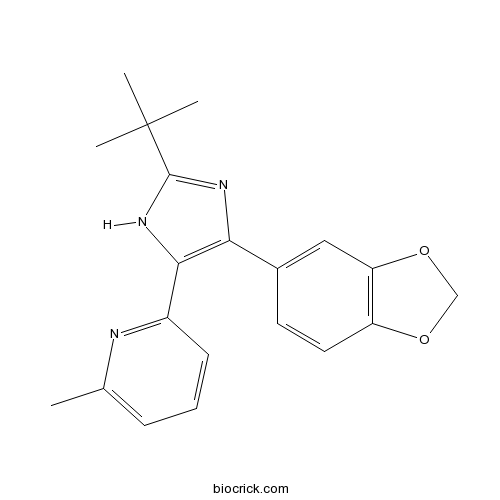
| Chemical Name | 2-[4-(1,3-benzodioxol-5-yl)-2-tert-butyl-1H-imidazol-5-yl]-6-methylpyridine | ||
| SMILES | CC1=CC=CC(=N1)C2=C(N=C(N2)C(C)(C)C)C3=CC4=C(C=C3)OCO4 | ||
| Standard InChIKey | WGZOTBUYUFBEPZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H21N3O2/c1-12-6-5-7-14(21-12)18-17(22-19(23-18)20(2,3)4)13-8-9-15-16(10-13)25-11-24-15/h5-10H,11H2,1-4H3,(H,22,23) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective inhibitor of transforming growth factor-β type I receptor (ALK5), ALK4 and ALK7 (IC50 values are 47 and 129 nM for ALK5 and ALK4 respectively). Selectively inhibits signaling from TGF-β and activin; does not inhibit other ALK family members. |

SB505124 Dilution Calculator

SB505124 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9815 mL | 14.9076 mL | 29.8151 mL | 59.6303 mL | 74.5379 mL |
| 5 mM | 0.5963 mL | 2.9815 mL | 5.963 mL | 11.9261 mL | 14.9076 mL |
| 10 mM | 0.2982 mL | 1.4908 mL | 2.9815 mL | 5.963 mL | 7.4538 mL |
| 50 mM | 0.0596 mL | 0.2982 mL | 0.5963 mL | 1.1926 mL | 1.4908 mL |
| 100 mM | 0.0298 mL | 0.1491 mL | 0.2982 mL | 0.5963 mL | 0.7454 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SB505124 is a selective and small molecule inhibitor of transforming growth Factor- β type 1 receptors ALK4, ALK5 and ALK7 with the IC50 values of 129±11nM and 47±5nM for ALK4 and ALK5, respectively. [1].
SB505124 has been reported as a potent inhibitor of the in vitro kinase activity of ALK4, ALK5 and ALK7 for its substrate Smad3. In addition, SB505124 has been revealed to be a reversible ATP competitive inhibitor. Moreover, SB505124 has shown no toxicity to renal epithelial A498 cells at concentrations up to 100μM for 48 h. Furthermore, SB505124 has been demonstrated to restrain the TGF-β-induced phosphorylation of Smad2 in three cell lines (HepG2 human hepatoma cells, C2C12 mouse myoblasts and Mv1Lu mink lung cells) in a concentration-dependent manner [1].
References:
[1] DaCosta Byfield S1, Major C, Laping NJ, Roberts AB. SB-505124 is a selective inhibitor of transforming growth factor-beta type I receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2004 Mar;65(3):744-52.
- Amonafide
Catalog No.:BCC1249
CAS No.:69408-81-7
- Lappaol H
Catalog No.:BCN8415
CAS No.:69394-18-9
- Sulbactam sodium
Catalog No.:BCC4852
CAS No.:69388-84-7
- Schisanhenol
Catalog No.:BCN2508
CAS No.:69363-14-0
- Pendulone
Catalog No.:BCN8248
CAS No.:69359-09-7
- Boc-D-Thr(Bzl)-OH
Catalog No.:BCC3454
CAS No.:69355-99-3
- Galanthamine hydrochloride
Catalog No.:BCC8277
CAS No.:5072-47-9
- Obtucarbamate A
Catalog No.:BCN3936
CAS No.:6935-99-5
- 2-Benzylaminopyridine
Catalog No.:BCC8565
CAS No.:6935-27-9
- CPPHA
Catalog No.:BCC1501
CAS No.:693288-97-0
- CYC116
Catalog No.:BCC2181
CAS No.:693228-63-6
- H-Ile-OtBu.HCl
Catalog No.:BCC2965
CAS No.:69320-89-4
- 17-PA
Catalog No.:BCC7452
CAS No.:694438-95-4
- Parvifuran
Catalog No.:BCN7780
CAS No.:69470-93-5
- 2-Methyl-4-isobutyrylphloroglucinol
Catalog No.:BCN7176
CAS No.:69480-03-1
- Boc-D-His(Tos)-OH
Catalog No.:BCC3405
CAS No.:69541-68-0
- Thymopentin
Catalog No.:BCN8347
CAS No.:69558-55-0
- Octadecyl caffeate
Catalog No.:BCN6609
CAS No.:69573-60-0
- Tupichilignan A
Catalog No.:BCN4257
CAS No.:69586-96-5
- 4-Hydroxybenzylamine
Catalog No.:BCN1805
CAS No.:696-60-6
- CRT0044876
Catalog No.:BCC5109
CAS No.:6960-45-8
- Chamaejasmine
Catalog No.:BCN3132
CAS No.:69618-96-8
- H-D-Asp(OMe)-OMe.HCl
Catalog No.:BCC2897
CAS No.:69630-50-8
- H-D-Met-OMe.HCl
Catalog No.:BCC2998
CAS No.:69630-60-0
Blockade of ALK4/5 signaling suppresses cadmium- and erastin-induced cell death in renal proximal tubular epithelial cells via distinct signaling mechanisms.[Pubmed:30804470]
Cell Death Differ. 2019 Feb 25. pii: 10.1038/s41418-019-0307-8.
Various types of cell death, including apoptosis, necrosis, necroptosis, and ferroptosis, are induced in renal tubular epithelial cells following exposure to environmental stresses and toxicants such as osmotic stress, ischemia/reperfusion injury, cisplatin, and cadmium. This is known to cause renal dysfunction, but the cellular events preceding stress-induced cell death in renal tubules are not fully elucidated. The activin receptor-like kinase (ALK) 4/5, also known as activin-transforming growth factor (TGF) beta receptor, is involved in stress-induced renal injury. We, therefore, studied the role of ALK4/5 signaling in HK-2 human proximal tubular epithelial cell death induced by cisplatin, cadmium, hyperosmotic stress inducer, sorbitol, and the ferroptosis activator, erastin. We found that ALK4/5 signaling is involved in cadmium- and erastin-induced cell death, but not sorbitol- or cisplatin-induced apoptotic cell death. Cadmium exposure elevated the level of phosphorylated Smad3, and treatment with the ALK4/5 kinase inhibitors, SB431542 or SB505124, suppressed cadmium-induced HK-2 cell death. Cadmium-induced cell death was attenuated by siRNA-mediated ALK4 or Smad3 silencing, or by treatment with SIS3, a selective inhibitor of TGFbeta1-dependent Smad3 phosphorylation. Furthermore, ALK4/5 signaling activated Akt signaling to promote cadmium-induced HK-2 cell death. In contrast, siRNA-mediated Inhibin-bA silencing or treatment with TGFbeta1 or activin A had little effect on cadmium-induced HK-2 cell death. On the other hand, treatment with SB431542 or SB505124 attenuated erastin-induced ferroptosis by hyperactivating Nrf2 signaling in HK-2 cells. These results suggest that blockade of ALK4/5 signaling protects against cadmium- and erastin-induced HK-2 cell death via Akt and Nrf2 signaling pathways, respectively.
Regulation of MMP and TIMP expression in synovial fibroblasts from knee osteoarthritis with flexion contracture using adenovirus-mediated relaxin gene therapy.[Pubmed:30770167]
Knee. 2019 Feb 13. pii: S0968-0160(19)30009-2.
PURPOSE: The aim of this study was to investigate the effects of relaxin (RLN) expression on fibrosis inhibition in synovial fibroblasts. MATERIALS AND METHODS: Tissue cells from patients with knee osteoarthritis and >30 degrees flexion contractures were utilised. Synovial fibroblasts were activated by TGF-beta1 (two nanograms per millilitre) and then exposed to Ad-RLN as a therapeutic gene, adenovirus-lacZ construct as a marker gene, and SB505124 as an inhibitor for TGF-beta1 signal for 48h. The mRNA expression levels of collagens and MMPs were analysed by reverse transcription-polymerase chain reaction. Also, fibronectin, phosphorylation of Smad2 and ERK1/2, alpha smooth muscle actin, TIMP-1, TIMP-2, MMP-1 and MMP-13 levels were estimated using western blotting, and the total collagen synthesis was assayed. RESULTS: Ad-RLN-transduced synovial fibroblasts demonstrated 17%, 13%, and 48% reduction in collagen I, III and IV mRNA expression levels, respectively, and a 40% decrease in MMP-3, MMP-8, 20% decrease in MMP-9, MMP-13 mRNA expression, compared to non-Ad-RLN-transduced cells. In protein expression, Ad-RLN-transduced synovial fibroblasts demonstrated 46% increase in MMP-1, 5% decrease in MMP-2, 51% increase in MMP-9, and 22% increase in MMP-13, compared to non-Ad-RLN-transduced cells. Ad-RLN-transduced synovial fibroblasts showed a 25% decrease in TIMP-1 and 65% decrease in TIMP-2 protein expression at 48h, compared to non-Ad-RLN-transduced cells. Ad-RLN-transduced synovial fibroblasts demonstrated a 45% inhibition of fibronectin in protein expression level and 38% decrease in total collagen synthesis at 48h, compared to non-Ad-RLN-transduced cells. CONCLUSION: Relaxin expression exerted anti-fibrogenic effects on synovial fibroblasts from patients with knee osteoarthritis and flexion contractures. Therefore, relaxin could be an alternative therapeutic agent during the initial stage of osteoarthritis with flexion contracture by exerting its anti-fibrogenic effects.
ACVR1C/SMAD2 signaling promotes invasion and growth in retinoblastoma.[Pubmed:30401983]
Oncogene. 2019 Mar;38(12):2056-2075.
Retinoblastoma is the most common intraocular cancer in children. While the primary tumor can often be treated by local or systemic chemotherapy, metastatic dissemination is generally resistant to therapy and remains a leading cause of pediatric cancer death in much of the world. In order to identify new therapeutic targets in aggressive tumors, we sequenced RNA transcripts in five snap frozen retinoblastomas which invaded the optic nerve and five which did not. A three-fold increase was noted in mRNA levels of ACVR1C/ALK7, a type I receptor of the TGF-beta family, in invasive retinoblastomas, while downregulation of DACT2 and LEFTY2, negative modulators of the ACVR1C signaling, was observed in most invasive tumors. A two- to three-fold increase in ACVR1C mRNA was also found in invasive WERI Rb1 and Y79 cells as compared to non-invasive cells in vitro. Transcripts of ACVR1C receptor and its ligands (Nodal, Activin A/B, and GDF3) were expressed in six retinoblastoma lines, and evidence of downstream SMAD2 signaling was present in all these lines. Pharmacological inhibition of ACVR1C signaling using SB505124, or genetic downregulation of the receptor using shRNA potently suppressed invasion, growth, survival, and reduced the protein levels of the mesenchymal markers ZEB1 and Snail. The inhibitory effects on invasion, growth, and proliferation were recapitulated by knocking down SMAD2, but not SMAD3. Finally, in an orthotopic zebrafish model of retinoblastoma, a 55% decrease in tumor spread was noted (p = 0.0026) when larvae were treated with 3 microM of SB505124, as compared to DMSO. Similarly, knockdown of ACVR1C in injected tumor cells using shRNA also resulted in a 54% reduction in tumor dissemination in the zebrafish eye as compared to scrambled shRNA control (p = 0.0005). Our data support a role for the ACVR1C/SMAD2 pathway in promoting invasion and growth of retinoblastoma.
Fibroblast-matrix interplay: Nintedanib and pirfenidone modulate the effect of IPF fibroblast-conditioned matrix on normal fibroblast phenotype.[Pubmed:29532550]
Respirology. 2018 Aug;23(8):756-763.
BACKGROUND AND OBJECTIVE: Idiopathic pulmonary fibrosis (IPF) is a progressive lung disease with poor prognosis. Activated fibroblasts are the key effector cells in fibrosis, producing excessive amounts of collagen and extracellular matrix (ECM) proteins. Whether the ECM conditioned by IPF fibroblasts determines the phenotype of naive fibroblasts is difficult to explore. METHODS: IPF-derived primary fibroblasts were cultured on Matrigel and then cleared using ammonium hydroxide, creating an IPF-conditioned matrix (CM). Normal fibroblast CM served as control. Normal fibroblasts were cultured on both types of CM, and cell count, cell distribution and markers of myofibroblast differentiation; transforming growth factor beta (TGFbeta) signalling; and ECM expression were assessed. The effects of the anti-fibrotic drugs nintedanib and pirfenidone at physiologically relevant concentrations were also explored. RESULTS: Normal fibroblasts cultured on IPF-CM arranged in large aggregates as a result of increased proliferation and migration. Moreover, increased levels of pSmad3, pSTAT3 (phospho signal transducer and activator of transcription 3), alpha smooth muscle actin (alphaSMA) and Collagen1a were found, suggesting a differentiation towards a myofibroblast-like phenotype. SB505124 (10 mumol/L) partially reversed these alterations, suggesting a TGFbeta contribution. Furthermore, nintedanib at 100 nmol/L and, to a lesser extent, pirfenidone at 100 mumol/L prevented the IPF-CM-induced fibroblast phenotype alterations, suggesting an attenuation of the ECM-fibroblast interplay. CONCLUSION: IPF fibroblasts alter the ECM, thus creating a CM that further propagates an IPF-like phenotype in normal fibroblasts. This assay demonstrated differences in drug activities for approved IPF drugs at clinically relevant concentrations. Thus, the matrix-fibroblast phenotype interplay might be a relevant assay to explore drug candidates for IPF treatment.
Rescue of a cherubism bone marrow stromal culture phenotype by reducing TGFbeta signaling.[Pubmed:29530719]
Bone. 2018 Jun;111:28-35.
We utilized a bone marrow stromal culture system to investigate changes in TGFbeta signaling in a mouse model for cherubism (Sh3bp2(KI/KI)). Interestingly, bone marrow cultures derived from cherubism mice not only displayed impaired osteoblast differentiation, but also had spontaneous osteoclast formation. PAI1, a target gene of TGFbeta signaling, was elevated 2-fold in cherubism CD11b(-),CD45(-) cells compared to wild type cells, while the expression of BAMBI, an inhibitor of TGFbeta signaling, was down-regulated. We also discovered that treatment of cherubism cultures with antagonists of the TGFbeta signaling pathway could largely rescue osteoblast differentiation and markedly reduce spontaneous osteoclast formation. Treatment with the type I TGFbeta receptor small molecule inhibitor SB505124 increased osteoblast reporter gene Col1a1-2.3 expression 24-fold and increased the expression of osteoblast gene markers Osterix (Sp7) 25-fold, Bone Sialoprotein (BSP) 7-fold, Osteocalcin (Bglap1) 100-fold, and Dentin Matrix Protein 1 (DMP1) 35-fold. In contrast, SB505124 treatment resulted in a significant reductions in osteoclast number and size. Gene expression analyses for RANKL, a positive regulator of osteoclast formation was 2.5-fold higher in osteoblast cultures derived from Sh3bp2(KI/KI) mice compared to wild type cultures, whereas OPG, an inhibitor of RANKL was 5-fold lower. However, SB505124 treatment reduced RANKL almost back down to wild type levels, while increasing OPG expression. Our studies also implicate a role for TGFbeta ligands in the etiology of cherubism. Blocking of TGFbeta ligands with the monoclonal antibody 1D11 increased Col1a1-2.3 reporter expression 4-fold and 13-fold in cultures derived from Sh3bp2(KI/+) and Sh3bp2(KI/KI) mice, respectively. Serum levels of latent TGFbeta1 were also 2-fold higher in SH3BP2(KI/KI) mice compared to wild type littermates. Taken together, these studies provide evidence that elevated levels of TGFbeta signaling may contribute to the disease phenotype of cherubism and a reduction in pathway activity may be an effective therapeutic approach to treat this rare disease.
Gremlin Regulates Podocyte Apoptosis via Transforming Growth Factor-beta (TGF-beta) Pathway in Diabetic Nephropathy.[Pubmed:29315280]
Med Sci Monit. 2018 Jan 9;24:183-189.
BACKGROUND Gremlin has been reported to be up-regulated in glomerular mesangial cells in diabetic nephropathy (DN). However, the regulation of gremlin in podocytes is still rarely reported. This study aimed to investigate the underlying mechanisms by which gremlin mediates the pathogenesis of DN via transforming growth factor-beta (TGF-beta) signaling pathways. MATERIAL AND METHODS Lentiviral and RNAi transfection were performed to increase and decrease gremlin expression in high-glucose conditions. Expression at the mRNA and protein level was detected by RT-qPCR and Western blotting. RESULTS The expression of gremlin was significantly higher in high-glucose (HG, 30mM) than normal-glucose (NG, 5.5 mM) conditions. The gremlin overexpression significantly suppressed the expression of nephrin and synaptopodin. The phosphorylation of canonical TGF-b signaling pathway components, including Smad2/3 and MKK, was increased in the gremlin-overexpressing group. In addition, the expression levels of Bax and cleaved caspase-3 were also higher in the gremlin-overexpressing group. TGF-beta pathway inhibitor (SB505124) significantly inhibited TGF-beta pathway activity and enhanced the expression of nephrin and synaptopodin. CONCLUSIONS These results indicate that gremlin can aggravate podocyte lesions through the TGF-beta signaling pathway, providing a novel therapeutic target for DN.
Age-Dependent Effects of ALK5 Inhibition and Mechanism of Neuroprotection in Neonatal Hypoxic-Ischemic Brain Injury.[Pubmed:28628913]
Dev Neurosci. 2017;39(1-4):338-351.
Neonatal encephalopathy due to hypoxic-ischemic (HI) brain injury triggers a wave of neuroinflammatory events attributed to causing the progressive degeneration and functional deficits seen weeks after the initial insult. In a recent set of studies, we evaluated the therapeutic efficacy of a small molecule antagonist for ALK5 (activin-like kinase 5 ), TGF-beta receptor in a rat model of moderate perinatal HI and found significant improvements in neurologic outcomes. Here, we have extended those studies to evaluate the efficacy of delayed TGF-beta receptor antagonism on postnatal day (P) 6 and P9 HI rat pups with and without hypothermia. The ALK5 receptor antagonist SB505124 was administered systemically by osmotic pump beginning 3 days following HI. Extending our earlier data set that showed protection of the hippocampus in P6 pups treated with SB505124, these animals sustained less damage to their hippocampi and had improved performance on the Morris water maze (MWM) when tested on P60 versus vehicle-treated HI animals. By contrast, SB505124 did not improve sensorimotor deficits and exacerbated hippocampal and thalamic volume loss when administered 3 days after HI to P9 pups. SB505124-treated rats injured on P9 tended to perform worse than their vehicle-treated counterparts on MWM, and SB505124 treatment did not preserve hippocampal or thalamic neurons in P9 pups when combined with hypothermia. To elucidate the mechanism whereby ALK5 inhibition reduced neuronal death in the P6 HI model, we assessed levels of autophagy markers in neurons of the neocortex, hippocampus, and thalamus, and in the subcortical white matter, and found that SB505124 increased numbers of autophagosomes and levels of lipidated LC3 (light chain 3), a key protein known to mediate autophagy. Altogether, our results demonstrate that there is a dynamic switch in the CNS response to TGF-beta1 that occurs around P9 in rats where TGF-beta signaling inhibition worsens functional outcomes. This response is similar to the outcome of antagonizing TGF-beta signaling in adult stroke and other CNS disease models. We conclude that attenuating TGF-beta1 signaling will likely be an effective treatment for HI-related encephalopathy in moderately preterm infants, offering protection of the neocortex, hippocampus, and thalamus with enhanced cerebral autophagy contributing to the decrease in the extent of progressive neuronal cell death.
Connective tissue growth factor mediates growth differentiation factor 8-induced increase of lysyl oxidase activity in human granulosa-lutein cells.[Pubmed:27392496]
Mol Cell Endocrinol. 2016 Oct 15;434:186-98.
Lysyl oxidase (LOX) is an essential enzyme for the stabilization of the extracellular matrix (ECM) and the subsequent follicle and oocyte maturation. Currently, there is limited information pertaining to the regulation of LOX activity in human ovarian tissue. Growth differentiation factor 8 (GDF8) is a unique member of the transforming growth factor-beta superfamily that is expressed in human granulosa cells and has important roles in regulating a variety of ovarian functions. The aim of the present study was to investigate the effects of GDF8 on the regulation of LOX expression and activity in human granulosa cells and to examine the underlying molecular determinants. An established immortalized human granulosa cell line (SVOG) and primary granulosa-lutein cells were used as study models. Using dual inhibition approaches (TGF-beta type I inhibitor SB505124 and small interfering RNAs) and ChIP analyses, we have demonstrated that GDF8 up-regulated the expression of connective tissue growth factor (CTGF) through the activin receptor-like kinase 5-mediated SMAD2/3-SMAD4 signaling pathways. In addition, the increase in CTGF expression contributed to the GDF8-induced increase in LOX expression and activity. Our findings suggest that GDF8 and CTGF may play critical roles in the regulation of ECM formation in human granulosa cells.
Epigenetic Library Screen Identifies Abexinostat as Novel Regulator of Adipocytic and Osteoblastic Differentiation of Human Skeletal (Mesenchymal) Stem Cells.[Pubmed:27194745]
Stem Cells Transl Med. 2016 Aug;5(8):1036-47.
UNLABELLED: : The epigenetic mechanisms promoting lineage-specific commitment of human skeletal (mesenchymal or stromal) stem cells (hMSCs) into adipocytes or osteoblasts are still not fully understood. Herein, we performed an epigenetic library functional screen and identified several novel compounds, including abexinostat, which promoted adipocytic and osteoblastic differentiation of hMSCs. Using gene expression microarrays, chromatin immunoprecipitation for H3K9Ac combined with high-throughput DNA sequencing (ChIP-seq), and bioinformatics, we identified several key genes involved in regulating stem cell proliferation and differentiation that were targeted by abexinostat. Concordantly, ChIP-quantitative polymerase chain reaction revealed marked increase in H3K9Ac epigenetic mark on the promoter region of AdipoQ, FABP4, PPARgamma, KLF15, CEBPA, SP7, and ALPL in abexinostat-treated hMSCs. Pharmacological inhibition of focal adhesion kinase (PF-573228) or insulin-like growth factor-1R/insulin receptor (NVP-AEW51) signaling exhibited significant inhibition of abexinostat-mediated adipocytic differentiation, whereas inhibition of WNT (XAV939) or transforming growth factor-beta (SB505124) signaling abrogated abexinostat-mediated osteogenic differentiation of hMSCs. Our findings provide insight into the understanding of the relationship between the epigenetic effect of histone deacetylase inhibitors, transcription factors, and differentiation pathways governing adipocyte and osteoblast differentiation. Manipulating such pathways allows a novel use for epigenetic compounds in hMSC-based therapies and tissue engineering. SIGNIFICANCE: This unbiased epigenetic library functional screen identified several novel compounds, including abexinostat, that promoted adipocytic and osteoblastic differentiation of human skeletal (mesenchymal or stromal) stem cells (hMSCs). These data provide new insight into the understanding of the relationship between the epigenetic effect of histone deacetylase inhibitors, transcription factors, and differentiation pathways controlling adipocyte and osteoblast differentiation of hMSCs. Manipulating such pathways allows a novel use for epigenetic compounds in hMSC-based therapies for tissue engineering, bone disease, obesity, and metabolic-disorders.
The essential role of fibroblasts in esophageal squamous cell carcinoma-induced angiogenesis.[Pubmed:18439605]
Gastroenterology. 2008 Jun;134(7):1981-93.
BACKGROUND & AIMS: Esophageal squamous cell carcinoma (ESCC) is known to be a highly angiogenic tumor. Here, we investigated the role of the stromal fibroblasts in the ESCC-induced angiogenic response using a novel 3-dimensional model. METHODS: A novel assay was developed where cocultures of ESCC and esophageal fibroblasts induced human microvascular endothelial cell (HMVEC) vascular network formation in a 3-dimensional collagen gel. Biochemical studies showed that the ESCC-induced activation of the fibroblasts was required to induce vascular network formation via a transforming growth factor (TGF)-beta and vascular endothelial growth factor (VEGF)-dependent pathway. RESULTS: Conditioned media from a panel of 4 ESCC lines transdifferentiated normal esophageal fibroblasts into myofibroblasts via TGF-beta signaling. The presence of fibroblasts was essential for efficient HMVEC network formation, and the addition of ESCC cells to these cultures greatly enhanced the angiogenic process. The role of TGF-beta in this process was shown by the complete inhibition of network formation following TGF-beta inhibitor treatment. Finally, we showed that ESCC-derived TGF-beta regulates angiogenesis through the release of VEGF from the fibroblasts and that the VEGF release was blocked following TGF-beta inhibition. CONCLUSIONS: This study shows the essential role of fibroblasts in the ESCC angiogenic-induced response and suggests that the pharmacologic targeting of the TGF-beta signaling axis could be of therapeutic benefit in this deadly disease.
Reactive oxygen species production via NADPH oxidase mediates TGF-beta-induced cytoskeletal alterations in endothelial cells.[Pubmed:16159901]
Am J Physiol Renal Physiol. 2005 Oct;289(4):F816-25.
Cytoskeletal alterations in endothelial cells have been linked to nitric oxide generation and cell-cell interactions. Transforming growth factor (TGF)-beta has been described to affect cytoskeletal rearrangement in numerous cell types; however, the underlying pathway is unclear. In the present study, we found that human umbilical vein endothelial cells (HUVEC) have marked cytoskeletal alterations with short-term TGF-beta treatment resulting in filipodia formation and F-actin assembly. The cytoskeletal alterations were blocked by the novel TGF-beta type I receptor/ALK5 kinase inhibitor (SB-505124) but not by the p38 kinase inhibitor (SB-203580). TGF-beta also induced marked stimulation of reactive oxygen species (ROS) within 5 min of TGF-beta exposure. TGF-beta stimulation of ROS was mediated by the NAPDH oxidase homolog Nox4 as DPI, an inhibitor of NADPH oxidase, and dominant-negative Nox4 adenovirus blocked ROS production. Finally, inhibition of ROS with ROS scavengers or dominant-negative Nox4 blocked the TGF-beta effect on cytoskeleton changes in endothelial cells. In conclusion, our studies show for the first time that TGF-beta-induced ROS production in human endothelial cells is via Nox4 and that TGF-beta alteration of cytoskeleton in HUVEC is mediated via a Nox4-dependent pathway.
SB-505124 is a selective inhibitor of transforming growth factor-beta type I receptors ALK4, ALK5, and ALK7.[Pubmed:14978253]
Mol Pharmacol. 2004 Mar;65(3):744-52.
Clinically, there is a great need for small molecule inhibitors that could control pathogenic effects of transforming growth factor (TGF-beta) and/or modulate effects of TGF-beta in normal responses. Inhibition of TGF-beta signaling would be predicted to enhance re-epithelialization of cutaneous wounds and reduce scarring fibrosis. Selective small molecule inhibitors of the TGF-beta signaling pathway developed for therapeutics will also be powerful tools in experimentally dissecting this complex pathway, especially its cross-talk with other signaling pathways. In this study, we characterized 2-(5-benzo[1,3]dioxol-5-yl-2-tert-butyl-3H-imidazol-4-yl)-6-methylpyridine hydrochloride (SB-505124), a member of a new class of small molecule inhibitors related to imidazole inhibitors of p38, which inhibit the TGF-beta type I receptor serine/threonine kinase known as activin receptor-like kinase (ALK) 5. We demonstrate that this compound selectively and concentration-dependently inhibits ALK4-, ALK5-, and ALK 7-dependent activation of downstream cytoplasmic signal transducers, Smad2 and Smad3, and of TGF-beta-induced mitogen-activated protein kinase pathway components but does not alter ALK1, ALK2, ALK3 or ALK6-induced Smad signaling. SB-505124 also blocks more complex endpoints of TGF-beta action, as evidenced by its ability to abrogate cell death caused by TGF-beta1 treatment. SB-505124 is three to five times more potent than a related ALK5 inhibitor described previously, SB-431542.


