Obtucarbamate ACAS# 6935-99-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6935-99-5 | SDF | Download SDF |
| PubChem ID | 235307 | Appearance | Powder |
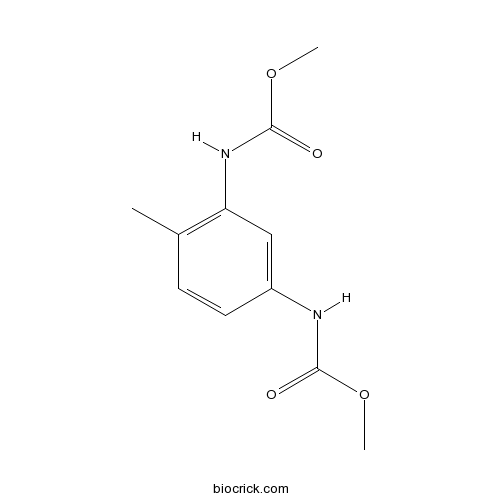
| Formula | C11H14N2O4 | M.Wt | 238.2 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl N-[3-(methoxycarbonylamino)-4-methylphenyl]carbamate | ||
| SMILES | CC1=C(C=C(C=C1)NC(=O)OC)NC(=O)OC | ||
| Standard InChIKey | CNPWIVIIZHULCN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H14N2O4/c1-7-4-5-8(12-10(14)16-2)6-9(7)13-11(15)17-3/h4-6H,1-3H3,(H,12,14)(H,13,15) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Obtucarbamate A has antitussive activity. 2. Obtucarbamate A exhibits significant inhibitory effect against neuroinflammation with the IC50 value of 10.57 uM . |
| Targets | NO |

Obtucarbamate A Dilution Calculator

Obtucarbamate A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1982 mL | 20.9908 mL | 41.9815 mL | 83.9631 mL | 104.9538 mL |
| 5 mM | 0.8396 mL | 4.1982 mL | 8.3963 mL | 16.7926 mL | 20.9908 mL |
| 10 mM | 0.4198 mL | 2.0991 mL | 4.1982 mL | 8.3963 mL | 10.4954 mL |
| 50 mM | 0.084 mL | 0.4198 mL | 0.8396 mL | 1.6793 mL | 2.0991 mL |
| 100 mM | 0.042 mL | 0.2099 mL | 0.4198 mL | 0.8396 mL | 1.0495 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-Benzylaminopyridine
Catalog No.:BCC8565
CAS No.:6935-27-9
- CPPHA
Catalog No.:BCC1501
CAS No.:693288-97-0
- CYC116
Catalog No.:BCC2181
CAS No.:693228-63-6
- H-Ile-OtBu.HCl
Catalog No.:BCC2965
CAS No.:69320-89-4
- (R)-baclofen
Catalog No.:BCC4503
CAS No.:69308-37-8
- 12-Aminolauric acid
Catalog No.:BCC8436
CAS No.:693-57-2
- 3-beta-O-(cis-p-Coumaroyl)maslinic acid
Catalog No.:BCN1375
CAS No.:69297-40-1
- 8-O-Acetylharpagide
Catalog No.:BCN4256
CAS No.:6926-14-3
- Harpagide
Catalog No.:BCN4996
CAS No.:6926-08-5
- N-Benzylcinchonidinium chloride
Catalog No.:BCC9094
CAS No.:69257-04-1
- 1,2,3-Tri-O-methyl-7,8-methyleneflavellagic acid
Catalog No.:BCN7205
CAS No.:69251-99-6
- Pinoresinol 4-O-beta-D-glucopyranoside
Catalog No.:BCN1376
CAS No.:69251-96-3
- Galanthamine hydrochloride
Catalog No.:BCC8277
CAS No.:5072-47-9
- Boc-D-Thr(Bzl)-OH
Catalog No.:BCC3454
CAS No.:69355-99-3
- Pendulone
Catalog No.:BCN8248
CAS No.:69359-09-7
- Schisanhenol
Catalog No.:BCN2508
CAS No.:69363-14-0
- Sulbactam sodium
Catalog No.:BCC4852
CAS No.:69388-84-7
- Lappaol H
Catalog No.:BCN8415
CAS No.:69394-18-9
- Amonafide
Catalog No.:BCC1249
CAS No.:69408-81-7
- SB505124
Catalog No.:BCC5087
CAS No.:694433-59-5
- 17-PA
Catalog No.:BCC7452
CAS No.:694438-95-4
- Parvifuran
Catalog No.:BCN7780
CAS No.:69470-93-5
- 2-Methyl-4-isobutyrylphloroglucinol
Catalog No.:BCN7176
CAS No.:69480-03-1
- Boc-D-His(Tos)-OH
Catalog No.:BCC3405
CAS No.:69541-68-0
[Antitussive constituents of Disporum cantoniense].[Pubmed:24791497]
Zhongguo Zhong Yao Za Zhi. 2013 Dec;38(23):4099-103.
The antitussive activity assay for the root extraction of Disporum cantoniense was carried out with coughing mice induced by ammonia liquor. The results showed that the ethanol and water extractions of D. cantoniense possess strong antitussive activity, and the high dose of the former was better than positive control, and then the constituents of the ethanol extraction were separated and purified by various modern chromatographic techniques. Their structures were identified by physico-chemical properties and spectroscopic data. As a result, eight compounds were isolated and identified as stigmast-4-en-3-one(1), (22E, 24R)-ergosta-5, 7, 22-trien-3beta-ol(2), Obtucarbamate A(3), obtucarbamate B(4), neotigogenin(5), azo-2, 2'-bis[Z-(2,3-dihydroxy-4-methyl-5-methoxy) phenyl ethylene] (6),dimethyl {[carbonylbis (azanediyl)] bis( 2-methyl-5, 1-phenylene) j dicarbamate (7) , and quercetin-3-O-pB-D-glucopyranoside(8). All compounds were isolated from this plant for the first time, and the result of bioactivity-directed isolation showed that compounds 3, 4, and 6 had obvious effect on antitussive activity, and compound 6 had the same level as positive control.
Biotransformation of isofraxetin-6-O-beta-d-glucopyranoside by Angelica sinensis (Oliv.) Diels callus.[Pubmed:27919656]
Bioorg Med Chem Lett. 2017 Jan 15;27(2):248-253.
Isofraxetin-6-O-beta-d-glucopyranoside, identified from traditional medicinal herbal Xanthoceras sorbifolia Bunge, has been demonstrated to be a natural neuroinflammatory inhibitor. In order to obtain more derivatives with potential anti-neuroinflammatory effects, biotransformation was carried out. According to the characteristics of coumarin skeleton, suspension cultures of Angelica sinensis (Oliv.) Diels callus (A. sinensis callus) were employed because of the presence of diverse phenylpropanoids biosynthetic enzymes. As a result, 15 products were yielded from the suspension cultures, including a new coumarin: 8'-dehydroxymethyl cleomiscosin A (1), together with 14 known compounds. Their structures were elucidated by extensive spectroscopic analysis. Furthermore, the biotransformed pathways were discussed. Among them, compound 13 was transformed from isofraxetin-6-O-beta-d-glucopyranoside, while compounds 1-6, 10-12, 14-15 were derived from the culture medium stimulated by the substrate. The biotransformation processes include hydroxylation, oxidation and esterification. Furthermore, their inhibitory effects on lipopolysaccharide (LPS)-activated nitric oxide (NO) production were evaluated in BV2 microglial cells. It is worth noting that, 1, 1'-methanediylbis(4-methoxybenzene) (3), obtucarbamates A (5), 2-nonyl-4-hydroxyquinoline N-oxide (10) and 1H-indole-3-carbaldehyde (11) exhibited significant inhibitory effect against neuroinflammation with IC50 values at 1.22, 10.57, 1.02 and 0.76muM respectively, much stronger than that of the positive control minocycline (IC50 35.82muM).


