PrudomestinCAS# 3443-28-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3443-28-5 | SDF | Download SDF |
| PubChem ID | 10404353 | Appearance | Yellow powder |
| Formula | C17H14O7 | M.Wt | 330.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Herbacetin 4',8-dimethyl ether; 3,5,7-Trihydroxy 4',8-dimethoxyflavone | ||
| Solubility | Soluble in DMSO and methan | ||
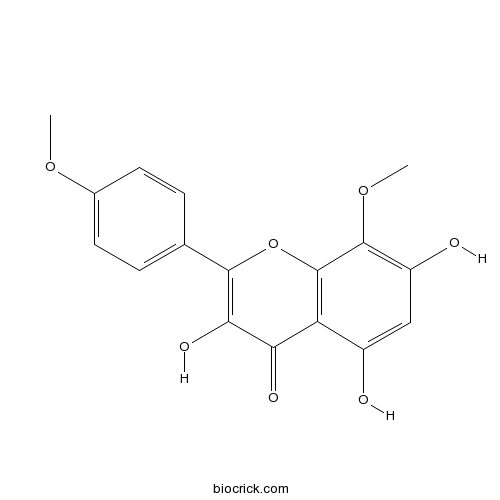
| Chemical Name | 3,5,7-trihydroxy-8-methoxy-2-(4-methoxyphenyl)chromen-4-one | ||
| SMILES | COC1=CC=C(C=C1)C2=C(C(=O)C3=C(O2)C(=C(C=C3O)O)OC)O | ||
| Standard InChIKey | HLSIOUXODPWHFI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H14O7/c1-22-9-5-3-8(4-6-9)15-14(21)13(20)12-10(18)7-11(19)16(23-2)17(12)24-15/h3-7,18-19,21H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard reference |
| Structure Identification | Tetrahedron,1966,22(3):941–948.A convenient synthesis of 3,5,7-trihydroxy-8-methoxy flavone, prudomestin and limocitrin[Reference: WebLink]
|

Prudomestin Dilution Calculator

Prudomestin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0276 mL | 15.1378 mL | 30.2755 mL | 60.551 mL | 75.6888 mL |
| 5 mM | 0.6055 mL | 3.0276 mL | 6.0551 mL | 12.1102 mL | 15.1378 mL |
| 10 mM | 0.3028 mL | 1.5138 mL | 3.0276 mL | 6.0551 mL | 7.5689 mL |
| 50 mM | 0.0606 mL | 0.3028 mL | 0.6055 mL | 1.211 mL | 1.5138 mL |
| 100 mM | 0.0303 mL | 0.1514 mL | 0.3028 mL | 0.6055 mL | 0.7569 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tirandamycin A
Catalog No.:BCN1861
CAS No.:34429-70-4
- Ligularine
Catalog No.:BCN2090
CAS No.:34429-54-4
- Ikshusterol
Catalog No.:BCN5278
CAS No.:34427-61-7
- Lyoniside
Catalog No.:BCN5277
CAS No.:34425-25-7
- 2,3-dihydrosciadopitysin
Catalog No.:BCN4034
CAS No.:34421-19-7
- Lathyrol
Catalog No.:BCN4963
CAS No.:34420-19-4
- 8-Chloroadenosine
Catalog No.:BCC7935
CAS No.:34408-14-5
- Boc-Lys(Z)-OSu
Catalog No.:BCC3418
CAS No.:34404-36-9
- H-D-Lys(Z)-OH
Catalog No.:BCC2678
CAS No.:34404-32-5
- Boc-D-Glu-OBzl
Catalog No.:BCC3394
CAS No.:34404-30-3
- Betamipron
Catalog No.:BCC8876
CAS No.:3440-28-6
- H-D-Pro-OH
Catalog No.:BCC3023
CAS No.:344-25-2
- Alpha-Onocerin diacetate
Catalog No.:BCN6700
CAS No.:34434-99-6
- Amycomycin
Catalog No.:BCN1824
CAS No.:344362-08-9
- Nortrachelogenin
Catalog No.:BCN5280
CAS No.:34444-37-6
- PJ34 hydrochloride
Catalog No.:BCC2210
CAS No.:344458-15-7
- PJ34
Catalog No.:BCC1865
CAS No.:344458-19-1
- 17-ODYA
Catalog No.:BCC6717
CAS No.:34450-18-5
- Leukadherin 1
Catalog No.:BCC6332
CAS No.:344897-95-6
- Araloside X
Catalog No.:BCN2467
CAS No.:344911-90-6
- SSR 69071
Catalog No.:BCC2369
CAS No.:344930-95-6
- Pseudoephedrine Hydrochloride; Threo-Ephedrine Hydrochloride
Catalog No.:BCC8241
CAS No.:345-78-8
- Myricanol triacetate
Catalog No.:BCN5281
CAS No.:34509-52-9
- 1,3,6-Trihydroxy-2,5-dimethoxyxanthone
Catalog No.:BCN7216
CAS No.:345287-92-5
Standardization and xanthine oxidase inhibitory potential of Zanthoxylum armatum fruits.[Pubmed:30342965]
J Ethnopharmacol. 2019 Feb 10;230:1-8.
ETHNOPHARMACOLOGICAL RELEVANCE: Tejovati (Zanthoxylum armatum DC; Family- Rutaceae) popularly known as toothache tree is widely distributed in sub-tropical Himalaya region. Traditionally, The Southeast Asian population of Indo-Nepal origin uses it to treat asthma, gout, pain, and inflammation. The Ayurvedic action of the plant includes the balancing of Vata-Kapha in the body. Which lead to various ailments related to the circulation of blood and water, digestion, immunity, and skin. Therefore, in-vitro xanthine oxidase (XO) inhibition potential of the extract could be worth to explore prospect in the prevention/treatment of gouty affections of the joints and other diseases. AIM OF STUDY: Anti-inflammatory and antioxidant potential of Z. armatum fruit (ZAF) has been reported. To date, no scientific study to validate the claim for gout treatment/management has been attempted so far. The present study deals with the xanthine oxidase inhibitory potential of a various extract of ZAF and marker-based high-performance liquid chromatography (HPLC) standardization of most active fraction. MATERIALS AND METHODS: Liquid-liquid partioning of crude methanol extract of the ZAF followed by repeated column chromatography of most active fraction has resulted in the isolation of seven compounds. Five distinct groups of compounds were isolated, purified, and identified. We have investigated the therapeutic action of ZAF in the management of gout through in-vitro assay of XO, a key enzyme involved in gout pathogenesis. RESULTS: Phytochemical investigation of ZAF has resulted in the isolation of seven compounds of diverse nature. It is noteworthy to mention that out of seven, five compounds have shown the xanthine oxidase inhibitory action. The ethyl acetate fraction was most potent to inhibit XO. The XO inhibitory activity (IC50 values) of isolated marker chemical was ranging from 5.62 to 41.21microM. Three compounds viz. acetyl phenyl acetate (ZA-2), Prudomestin (ZA-6), and tambulin (ZA-7) showed the most potent XO inhibitory activity (IC50 approximately 6microM) comparable with a positive control (Allopurinol, IC50, 3.38microM). This is the first validated HPLC-PDA method for simultaneous analysis and accurate quantification of seven compounds (phenolic acid, acetyl phenyl acetate, xylopyranoside, diphenyl ether and three flavones) in ZAF as well as their distribution in other tissues of the plant. CONCLUSION: Most potent three chemicals (ZA-2, 6 and 7) could be considered as bioactive to ensure the robust quality of the enriched fraction of ZAF with defined XO inhibition potential. Therefore, either single purified component or their enriched fraction could be a better choice for the management of gout than the crude extract of ZAF. Developed HPLC method is suitable for quality assurance analysis and process control of ZAF derived product intended for gout management. XO inhibitory potential exhibited by the characterized compounds validate the traditional use of this ZAF for the treatment of gout. Further, a detailed study is required to assess the effect of ZAF chemicals on serum uric acid and mechanism of XO inhibition.
Characterization and evaluation of bioactive polyphenolic constituents from Zanthoxylum armatum DC., a traditionally used plant.[Pubmed:28242546]
Biomed Pharmacother. 2017 May;89:366-375.
Zanthoxylum armatum or Timoor has been used in different traditional system of medicine due to its aromatic properties and also in the treatment of cancer, diarrhea and cholera. In the present investigation, four chemically distinct compounds namely Tambulin (6), Prudomestin (7), Ombuin (8) and 3, 4, 5, 3', 4', 5'-hexahydroxydiphenyl ether (9) have been isolated and quantified from the fruits. To explore the biological activities, we have further studied the antiproliferative, antimicrobial and antioxidant efficacy. Tambulin which was also found in maximum amount (0.125%) in fruits revealed significant antiproliferative activity (IC50 37.96+/-0.36 to 48.7+/-0.21mug/mL) against breast, liver, colon and skin cancer cell lines corroborated by resilient binding interaction with SDH (-6.76Kcal/mol) and inhibition constant (Ki: 11.02muM). Hexane and ethyl acetate fraction exhibited moderate antibacterial efficacy (MIC: 250-1000mug/mL) against selected pathogenic microbes while Ombuin displayed broad spectrum antibacterial effect with MIC ranges from 125 to 500mug/mL. Total phenolic content (5.27+/-0.06 to 46.12+/-0.40mg/g of gallic acid equivalents), total flavonoids content (6.05+/-0.24 to 14.46+/-0.73mg/g of quercetin equivalents), ferric reducing power (42.35+/-0.85 to 62.52+/-0.66mg/g of ferrous sulfate equivalents) and percent free radical scavenging activity (59.56+/-0.38 to 64.85+/-1.78) were also estimated. Our findings infer that Tambulin exhibited significant antiproliferative activity whereas Ombuin was found to display broad spectrum antibacterial activity which adds one more positive attribute to its traditional usage.


