2,3-dihydrosciadopitysinCAS# 34421-19-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 34421-19-7 | SDF | Download SDF |
| PubChem ID | 91886695 | Appearance | Yellow powder |
| Formula | C33H26O10 | M.Wt | 582.6 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
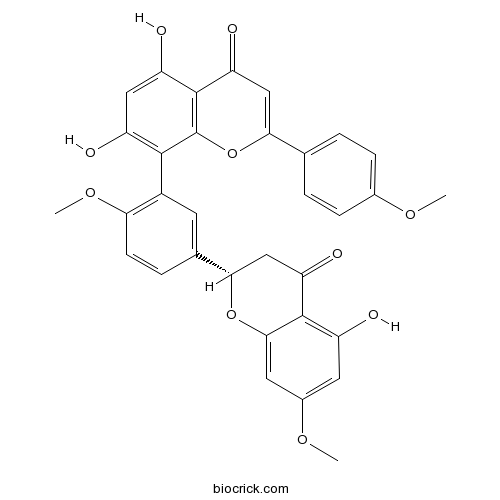
| Chemical Name | 5,7-dihydroxy-8-[5-[(2S)-5-hydroxy-7-methoxy-4-oxo-2,3-dihydrochromen-2-yl]-2-methoxyphenyl]-2-(4-methoxyphenyl)chromen-4-one | ||
| SMILES | COC1=CC=C(C=C1)C2=CC(=O)C3=C(O2)C(=C(C=C3O)O)C4=C(C=CC(=C4)C5CC(=O)C6=C(C=C(C=C6O5)OC)O)OC | ||
| Standard InChIKey | IHBQEDJQLPQAHW-NDEPHWFRSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard reference |
| In vitro | New constituent from Podocarpus macrophyllus var. macrophyllus shows anti-tyrosinase effect and regulates tyrosinase-related proteins and mRNA in human epidermal melanocytes.[Pubmed: 17473463]Chem Pharm Bull (Tokyo). 2007 May;55(5):757-61.A new biflavonoid, 2,3-dihydro-4',4'''-di-O-methylamentoflavone (5), and five known compounds, (-)-catechin (1), quercetin (2), 2,3-dihydrosciadopitysin (3), sciadopitysin (4), and isoginkgetin (6), were isolated from Podocarpus macrophyllus var. macrophyllus (Podocarpaceae). These compounds were evaluated their ability to inhibit cellular tyrosinase activity and for their melanin inhibitory activity in human epidermal melanocytes (HEMn). Antifungal activity of biflavones from Taxus baccata and Ginkgo biloba.[Pubmed: 12622229]Z Naturforsch C. 2003 Jan-Feb;58(1-2):65-9.Bilobetin and 4'''-O-methylamentoflavone were isolated and identified in the needles of Taxus baccata, for the first time in this species.
|

2,3-dihydrosciadopitysin Dilution Calculator

2,3-dihydrosciadopitysin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7164 mL | 8.5822 mL | 17.1644 mL | 34.3289 mL | 42.9111 mL |
| 5 mM | 0.3433 mL | 1.7164 mL | 3.4329 mL | 6.8658 mL | 8.5822 mL |
| 10 mM | 0.1716 mL | 0.8582 mL | 1.7164 mL | 3.4329 mL | 4.2911 mL |
| 50 mM | 0.0343 mL | 0.1716 mL | 0.3433 mL | 0.6866 mL | 0.8582 mL |
| 100 mM | 0.0172 mL | 0.0858 mL | 0.1716 mL | 0.3433 mL | 0.4291 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lathyrol
Catalog No.:BCN4963
CAS No.:34420-19-4
- 8-Chloroadenosine
Catalog No.:BCC7935
CAS No.:34408-14-5
- Boc-Lys(Z)-OSu
Catalog No.:BCC3418
CAS No.:34404-36-9
- H-D-Lys(Z)-OH
Catalog No.:BCC2678
CAS No.:34404-32-5
- Boc-D-Glu-OBzl
Catalog No.:BCC3394
CAS No.:34404-30-3
- Betamipron
Catalog No.:BCC8876
CAS No.:3440-28-6
- H-D-Pro-OH
Catalog No.:BCC3023
CAS No.:344-25-2
- N-Demethyl-alpha-obscurine
Catalog No.:BCN7362
CAS No.:34399-44-5
- Isovallesiachotamine
Catalog No.:BCN3549
CAS No.:34384-71-9
- Acebutolol HCl
Catalog No.:BCC4322
CAS No.:34381-68-5
- CP-673451
Catalog No.:BCC4981
CAS No.:343787-29-1
- Ginsenoside Ro
Catalog No.:BCN5937
CAS No.:34367-04-9
- Lyoniside
Catalog No.:BCN5277
CAS No.:34425-25-7
- Ikshusterol
Catalog No.:BCN5278
CAS No.:34427-61-7
- Ligularine
Catalog No.:BCN2090
CAS No.:34429-54-4
- Tirandamycin A
Catalog No.:BCN1861
CAS No.:34429-70-4
- Prudomestin
Catalog No.:BCN5279
CAS No.:3443-28-5
- Alpha-Onocerin diacetate
Catalog No.:BCN6700
CAS No.:34434-99-6
- Amycomycin
Catalog No.:BCN1824
CAS No.:344362-08-9
- Nortrachelogenin
Catalog No.:BCN5280
CAS No.:34444-37-6
- PJ34 hydrochloride
Catalog No.:BCC2210
CAS No.:344458-15-7
- PJ34
Catalog No.:BCC1865
CAS No.:344458-19-1
- 17-ODYA
Catalog No.:BCC6717
CAS No.:34450-18-5
- Leukadherin 1
Catalog No.:BCC6332
CAS No.:344897-95-6
New constituent from Podocarpus macrophyllus var. macrophyllus shows anti-tyrosinase effect and regulates tyrosinase-related proteins and mRNA in human epidermal melanocytes.[Pubmed:17473463]
Chem Pharm Bull (Tokyo). 2007 May;55(5):757-61.
A new biflavonoid, 2,3-dihydro-4',4'''-di-O-methylamentoflavone (5), and five known compounds, (-)-catechin (1), quercetin (2), 2,3-dihydrosciadopitysin (3), sciadopitysin (4), and isoginkgetin (6), were isolated from Podocarpus macrophyllus var. macrophyllus (Podocarpaceae). These compounds were evaluated their ability to inhibit cellular tyrosinase activity and for their melanin inhibitory activity in human epidermal melanocytes (HEMn). In the melanin synthesis assay, 2,3-dihydro-4',4'''-di-O-methylamentoflavone (5) showed a potent anti-tyrosinase effect with IC(50)=0.098 mM in HEMn. It also significantly decreased both protein and mRNA levels of the tyrosinase-related protein-2 (TRP-2) by Western blot and quantitative real-time PCR (qRT-PCR) analysis. These findings suggest that the new compound, 2,3-dihydro-4',4'''-di-O-methylamentoflavone (5), is the most active component of P. macrophyllus var. macrophyllus in inhibiting pigmentation and that this inhibition is exerted through inhibition of transcription of the genes encoding TRP2.
Antifungal activity of biflavones from Taxus baccata and Ginkgo biloba.[Pubmed:12622229]
Z Naturforsch C. 2003 Jan-Feb;58(1-2):65-9.
Bilobetin and 4'''-O-methylamentoflavone were isolated and identified in the needles of Taxus baccata, for the first time in this species. The antifungal activity of biflavones from T. baccata and Ginkgo biloba, namely amentoflavone, 7-O-methylamentoflavone, bilobetin, ginkgetin, sciadopitysin and 2,3-dihydrosciadopitysin towards the fungi Alternaria alternata, Fusarium culmorum, Cladosporium oxysporum was determined employing computer-aided image analysis couplet to a microscope. Bilobetin exhibited a significant antifungal activity with values of ED50 14, 11 and 17 microM respectively. This compound completely inhibited the growth of germinating tubes of Cladosporium oxysporum and Fusarium culmorum at a concentration 100 microM. Activity of ginkgetin and 7-O-methylamentoflavone towards Alternaria alternata was stronger than that of bilobetin. Moreover, slight structural changes in the cell wall of Alternaria alternata exposed to ginkgetin at concentration of 200 microM were observed.


