glucagon receptor antagonists 2Glucagon receptor antagonist,highly potent CAS# 202917-18-8 |
- Exendin-4
Catalog No.:BCC1568
CAS No.:141758-74-9
- glucagon receptor antagonists 3
Catalog No.:BCC1595
CAS No.:202917-17-7
- glucagon receptor antagonists 1
Catalog No.:BCC1593
CAS No.:503559-84-0
- MK 0893
Catalog No.:BCC1752
CAS No.:870823-12-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

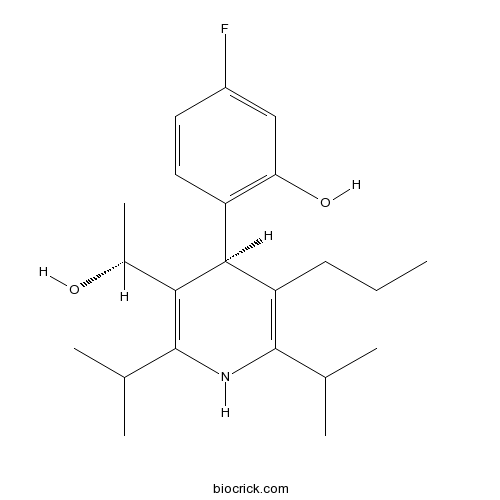
| Cas No. | 202917-18-8 | SDF | Download SDF |
| PubChem ID | 66577053 | Appearance | Powder |
| Formula | C22H30FNO2 | M.Wt | 359.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 16.67 mg/mL (46.37 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 5-fluoro-2-[(4S)-3-[(1R)-1-hydroxyethyl]-2,6-di(propan-2-yl)-5-propyl-1,4-dihydropyridin-4-yl]phenol | ||
| SMILES | CCCC1=C(NC(=C(C1C2=C(C=C(C=C2)F)O)C(C)O)C(C)C)C(C)C | ||
| Standard InChIKey | OINVVPOIGFSNHM-VLIAUNLRSA-N | ||
| Standard InChI | InChI=1S/C22H32FNO2/c1-7-8-17-20(16-10-9-15(23)11-18(16)26)19(14(6)25)22(13(4)5)24-21(17)12(2)3/h9-14,20,24-26H,7-8H2,1-6H3/t14-,20+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Glucagon receptor antagonists-2 is a highly potent glucagon receptor antagonist. References: | |||||

glucagon receptor antagonists 2 Dilution Calculator

glucagon receptor antagonists 2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7817 mL | 13.9086 mL | 27.8172 mL | 55.6344 mL | 69.543 mL |
| 5 mM | 0.5563 mL | 2.7817 mL | 5.5634 mL | 11.1269 mL | 13.9086 mL |
| 10 mM | 0.2782 mL | 1.3909 mL | 2.7817 mL | 5.5634 mL | 6.9543 mL |
| 50 mM | 0.0556 mL | 0.2782 mL | 0.5563 mL | 1.1127 mL | 1.3909 mL |
| 100 mM | 0.0278 mL | 0.1391 mL | 0.2782 mL | 0.5563 mL | 0.6954 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
highly potent glucagon receptor antagonist
- glucagon receptor antagonists 3
Catalog No.:BCC1595
CAS No.:202917-17-7
- 7-Benzyloxyindole
Catalog No.:BCC8778
CAS No.:20289-27-4
- 4-Benzyloxyindole
Catalog No.:BCC8700
CAS No.:20289-26-3
- 8-Hydroxy-3,5,7,3',4',5'-hexamethoxyflavone
Catalog No.:BCN1506
CAS No.:202846-95-5
- Rosmarinic acid
Catalog No.:BCN5893
CAS No.:20283-92-5
- Safinamide Mesylate
Catalog No.:BCC2320
CAS No.:202825-46-5
- Ralfinamide mesylate
Catalog No.:BCC7844
CAS No.:202825-45-4
- BMS 191011
Catalog No.:BCC7448
CAS No.:202821-81-6
- Licoagrochalcone A
Catalog No.:BCC8197
CAS No.:202815-28-9
- Orexin B (mouse)
Catalog No.:BCC5766
CAS No.:202801-92-1
- 4-Methoxycoumarine
Catalog No.:BCN6536
CAS No.:20280-81-3
- Homodihydrocapsaicin I
Catalog No.:BCN7844
CAS No.:20279-06-5
- Conantokin-R
Catalog No.:BCC5980
CAS No.:202925-60-8
- NF 340
Catalog No.:BCC7785
CAS No.:202982-98-7
- NF 279
Catalog No.:BCC6964
CAS No.:202983-32-2
- Aporheine
Catalog No.:BCN4802
CAS No.:2030-53-7
- Clofazimine
Catalog No.:BCC4651
CAS No.:2030-63-9
- Saponarin
Catalog No.:BCN2280
CAS No.:20310-89-8
- Solamargine
Catalog No.:BCN2305
CAS No.:20311-51-7
- Procyanidin B1
Catalog No.:BCN6314
CAS No.:20315-25-7
- Tiliroside
Catalog No.:BCN4889
CAS No.:20316-62-5
- Solamarine
Catalog No.:BCN3806
CAS No.:20318-30-3
- 3,5-Diacetamido-4-methylbenzoic acid
Catalog No.:BCN1505
CAS No.:6633-37-0
- 3,4,5-Trimethoxy-trans-cinnamic acid
Catalog No.:BCN3423
CAS No.:20329-98-0
Discovery of a Novel Series of Orally Bioavailable and CNS Penetrant Glucagon-like Peptide-1 Receptor (GLP-1R) Noncompetitive Antagonists Based on a 1,3-Disubstituted-7-aryl-5,5-bis(trifluoromethyl)-5,8-dihydropyrimido[4,5-d]pyrim idine-2,4(1H,3H)-dione Core.[Pubmed:28103022]
J Med Chem. 2017 Feb 23;60(4):1611-1616.
A duplexed, functional multiaddition high throughput screen and subsequent optimization effort identified the first orally bioavailable and CNS penetrant glucagon-like peptide-1 receptor (GLP-1R) noncompetitive antagonist. Antagonist 5d not only blocked exendin-4-stimulated insulin release in islets but also lowered insulin levels while increasing blood glucose in vivo.
Discovery of furan-2-carbohydrazides as orally active glucagon receptor antagonists.[Pubmed:25127101]
Bioorg Med Chem Lett. 2014 Sep 1;24(17):4266-70.
Furan-2-carbohydrazides were found as orally active glucagon receptor antagonists. Starting from the hit compound 5, we successfully determined the structure activity relationships of a series of derivatives obtained by modifying the acidity of the phenol. We identified the ortho-nitrophenol as a good scaffold for glucagon receptor inhibitory activity. Our efforts have led to the discovery of compound 7l as a potent glucagon receptor antagonist with good bioavailability and satisfactory long half-life.
Discovery of N-aryl-2-acylindole human glucagon receptor antagonists.[Pubmed:22030028]
Bioorg Med Chem Lett. 2011 Dec 1;21(23):7124-30.
A novel class of N-aryl-2-acylindole human glucagon receptor (hGCGR) antagonists is reported. These compounds demonstrate good pharmacokinetic profiles in multiple preclinical species. One compound from this series, indole 33, is orally active in a transgenic murine pharmacodynamic model. Furthermore, a 1mg/kg oral dose of indole 33 lowers ambient glucose levels in an ob/ob/hGCGR transgenic murine diabetes model. This compound was deemed suitable for preclinical safety studies and was found to be well tolerated in an 8-day experimental rodent tolerability study. The combination of preclinical efficacy and safety observed with compound 33 highlights the potential of this class as a treatment for type 2 diabetes.
Optimization of alkylidene hydrazide based human glucagon receptor antagonists. Discovery of the highly potent and orally available 3-cyano-4-hydroxybenzoic acid [1-(2,3,5,6-tetramethylbenzyl)-1H-indol-4-ylmethylene]hydrazide.[Pubmed:12477359]
J Med Chem. 2002 Dec 19;45(26):5755-75.
Highly potent human glucagon receptor (hGluR) antagonists have been prepared employing both medicinal chemistry and targeted libraries based on modification of the core (proximal) dimethoxyphenyl group, the benzyl ether linkage, as well as the (distal) benzylic aryl group of the lead 2, 3-cyano-4-hydroxybenzoic acid (3,5-dimethoxy-4-isopropylbenzyloxybenzylidene)hydrazide. Electron-rich proximal aryl moieties such as mono- and dimethoxy benzenes, naphthalenes, and indoles were found to be active. The SAR was found to be quite insensitive regarding the linkage to the distal aryl group, since long and short as well as polar and apolar linkers gave highly potent compounds. The presence of a distal aryl group was not crucial for obtaining high binding affinity to the hGluR. In many cases, however, the affinity could be further optimized with substituted distal aryl groups. Representative compounds have been tested for in vitro metabolism, and structure-metabolism relationships are described. These efforts lead to the discovery of 74, NNC 25-2504, 3-cyano-4-hydroxybenzoic acid [1-(2,3,5,6-tetramethylbenzyl)-1H-indol-4-ylmethylene]hydrazide, with low in vitro metabolic turnover. 74 was a highly potent noncompetitive antagonist of the human glucagon receptor (IC(50) = 2.3 nM, K(B) = 760 pM) and of the isolated rat receptor (IC(50) = 430 pM, K(B) = 380 pM). Glucagon-stimulated glucose production from isolated primary rat hepatocytes was inhibited competitively by 74 (K(i) = 14 nM). This compound was orally available in dogs (F(po) = 15%) and was active in a glucagon-challenged rat model of hyperglucagonemia and hyperglycemia.


