PiperitolCAS# 52151-92-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 52151-92-5 | SDF | Download SDF |
| PubChem ID | 10247670 | Appearance | Powder |
| Formula | C20H20O6 | M.Wt | 356.4 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
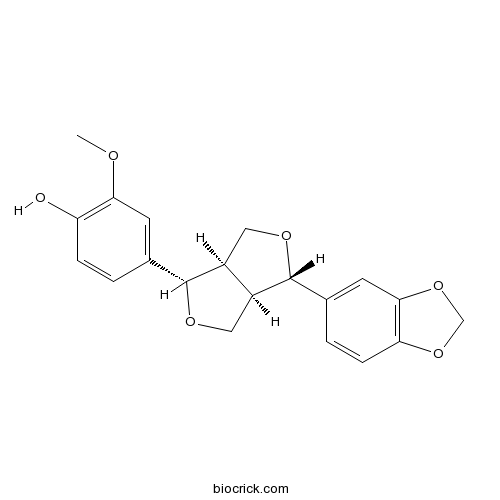
| Chemical Name | 4-[(3S,3aR,6S,6aR)-3-(1,3-benzodioxol-5-yl)-1,3,3a,4,6,6a-hexahydrofuro[3,4-c]furan-6-yl]-2-methoxyphenol | ||
| SMILES | COC1=C(C=CC(=C1)C2C3COC(C3CO2)C4=CC5=C(C=C4)OCO5)O | ||
| Standard InChIKey | VBIRCRCPHNUJAS-AFHBHXEDSA-N | ||
| Standard InChI | InChI=1S/C20H20O6/c1-22-17-6-11(2-4-15(17)21)19-13-8-24-20(14(13)9-23-19)12-3-5-16-18(7-12)26-10-25-16/h2-7,13-14,19-21H,8-10H2,1H3/t13-,14-,19+,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Piperitol has in vitro antioxidant potential, it also exhibits a stronger CNS depressant effect than that of M. rotundifolia. |
| In vitro | CNS activity of Mentha rotundifolia and Mentha longifolia essential oil in mice and rats.[Reference: WebLink]Phytotherapy Research, 2010, 4(6):232-234.
Identification of Methanol-Soluble Compounds in Sesame and Evaluation of Antioxidant Potential of Its Lignans.[Reference: WebLink]Journal of Agricultural and Food Chemistry, 2011, 59(7):3214-3219.The methanol extract of sesame (Sesamum indicum) seeds was fractionated and purified with the assistance of conventional column chromatography to afford 29 compounds including seven furofuran lignans. Among these isolates, (+)-samin (1) was obtained from the natural source for the first time. In addition, (-)-asarinin (30) and sesamol (31) were generated by oxidative derivation from (+)-sesamolin (2) and (+)-sesamin (3), two abundant lignans found in sesame seeds. |
| Structure Identification | Chinese Traditional and Herbal Drugs, 2009, 40(10):1536-1539.Chemical constituents in leaves of Piper laetispicum.[Reference: WebLink] To study the chemical constituents in the leaves of Piper laetispicum. |

Piperitol Dilution Calculator

Piperitol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8058 mL | 14.0292 mL | 28.0584 mL | 56.1167 mL | 70.1459 mL |
| 5 mM | 0.5612 mL | 2.8058 mL | 5.6117 mL | 11.2233 mL | 14.0292 mL |
| 10 mM | 0.2806 mL | 1.4029 mL | 2.8058 mL | 5.6117 mL | 7.0146 mL |
| 50 mM | 0.0561 mL | 0.2806 mL | 0.5612 mL | 1.1223 mL | 1.4029 mL |
| 100 mM | 0.0281 mL | 0.1403 mL | 0.2806 mL | 0.5612 mL | 0.7015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- H-Tyr(Bzl)-OBzl.HCl
Catalog No.:BCC3131
CAS No.:52142-01-5
- 3-O-Acetylpinobanksin
Catalog No.:BCN5660
CAS No.:52117-69-8
- 2,4-Dihydroxy-6-methoxy-3-formylacetophenone
Catalog No.:BCN1430
CAS No.:52117-67-6
- Karanjin
Catalog No.:BCN8370
CAS No.:521-88-0
- Broxyquinoline
Catalog No.:BCC4642
CAS No.:521-74-4
- Cinnamoylcocaine
Catalog No.:BCN1429
CAS No.:521-67-5
- Frangulin A
Catalog No.:BCC8174
CAS No.:521-62-0
- Physcion
Catalog No.:BCN5663
CAS No.:521-61-9
- Vulpic acid
Catalog No.:BCN6546
CAS No.:521-52-8
- Pedicin
Catalog No.:BCN4845
CAS No.:521-51-7
- Cannabinol
Catalog No.:BCN7968
CAS No.:521-35-7
- Sciadopitysin
Catalog No.:BCN5662
CAS No.:521-34-6
- 7-Hydroxy-2,3,4,5-tetrahydro-1H-benzofuro[2,3-c]azepin-1-one
Catalog No.:BCC3960
CAS No.:521937-07-5
- N'-Methylammodendrine
Catalog No.:BCN2147
CAS No.:52196-10-8
- Evoxine
Catalog No.:BCN5664
CAS No.:522-11-2
- Quercitrin
Catalog No.:BCN5665
CAS No.:522-12-3
- Deguelin
Catalog No.:BCN4804
CAS No.:522-17-8
- Norsanguinarine
Catalog No.:BCN3714
CAS No.:522-30-5
- Lochnerine
Catalog No.:BCN5667
CAS No.:522-47-4
- Tetrahydrozoline HCl
Catalog No.:BCC4339
CAS No.:522-48-5
- Dequalinium Chloride
Catalog No.:BCC4998
CAS No.:522-51-0
- Allo-Yohimbine
Catalog No.:BCN3487
CAS No.:522-94-1
- Tetrahydroberberine
Catalog No.:BCN2648
CAS No.:522-97-4
- Lamalbid
Catalog No.:BCN3750
CAS No.:52212-87-0
Formation of a Methylenedioxy Bridge in (+)-Epipinoresinol by CYP81Q3 Corroborates with Diastereomeric Specialization in Sesame Lignans.[Pubmed:30085233]
Plant Cell Physiol. 2018 Nov 1;59(11):2278-2287.
Plant specialized metabolites are often found as lineage-specific diastereomeric isomers. For example, Sesamum alatum accumulates the specialized metabolite (+)-2-episesalatin, a furofuran-type lignan with a characteristic diastereomeric configuration rarely found in other Sesamum spp. However, little is known regarding how diastereomeric specificity in lignan biosynthesis is implemented in planta. Here, we show that S. alatum CYP81Q3, a P450 orthologous to S. indicum CYP81Q1, specifically catalyzes methylenedioxy bridge (MDB) formation in (+)-epipinoresinol to produce (+)-pluviatilol. Both (+)-epipinoresinol and (+)-pluviatilol are putative intermediates of (+)-2-episesalatin based on their diastereomeric configurations. On the other hand, CYP81Q3 accepts neither (+)- nor (-)-pinoresinol as a substrate. This diastereomeric selectivity of CYP81Q3 is in clear contrast to that of CYP81Q1, which specifically converts (+)-pinoresinol to (+)-sesamin via (+)-Piperitol by the sequential formation of two MDBs but does not accept (+)-epipinoresinol as a substrate. Moreover, (+)-pinoresinol does not interfere with the conversion of (+)-epipinoresinol to (+)-pluviatilol by CYP81Q3. Amino acid substitution and CO difference spectral analyses show that polymorphic residues between CYP81Q1 and CYP81Q3 proximal to their putative substrate pockets are crucial for the functional diversity and stability of these two enzymes. Our data provide clues to understanding how the lineage-specific functional differentiation of respective biosynthetic enzymes substantiates the stereoisomeric diversity of lignan structures.
Application of off-line two-dimensional high-performance countercurrent chromatography on the chloroform-soluble extract of Cuscuta auralis seeds.[Pubmed:29450982]
J Sep Sci. 2018 May;41(10):2169-2177.
In this study, the chloroform-soluble extract of Cuscuta auralis was separated successfully using off-line two-dimensional high-performance countercurrent chromatography, yielding a gamma-pyrone, two alkaloids, a flavonoid, and four lignans. The first-dimensional countercurrent separation using a methylene chloride/methanol/water (11:6:5, v/v/v) system yielded three subfractions (fractions I-III). The second-dimensional countercurrent separations, conducted on fractions I-III using n-hexane/ethyl acetate/methanol/water/acetic acid (5:5:5:5:0, 3:7:3:7:0, and 1:9:1:9:0.01, v/v/v/v/v) systems, gave maltol (1), (-)-(13S)-cuscutamine (2), (+)-(13R)-cuscutamine (3), (+)-pinoresinol (4), (+)-epipinoresinol (5), kaempferol (6), Piperitol (7), and (9R)-hydroxy-d-sesamin (8). To the best of our knowledge, maltol was identified for the first time in Cuscuta species. Furthermore, this report details the first full assignment of spectroscopic data of two cuscutamine epimers, (-)-(13S)-cuscutamine and (+)-(13R)-cuscutamine.
A new furofuran lignan diglycoside and other secondary metabolites from the antidepressant extract of Castilleja tenuiflora Benth.[Pubmed:26197306]
Molecules. 2015 Jul 21;20(7):13127-43.
Castilleja tenuiflora has been used for the treatment of several Central Nervous System (CNS) diseases. Herein we report the antidepressant activity of the methanol extract from the leaves of this medicinal plant. The oral administration of MeOH extract (500 mg/kg) induced a significant (p < 0.05) decrement of the immobility parameter on Forced Swimming Test (FST) and an increment in the latency and duration of the hypnosis, induced by administration of sodium pentobarbital (Pbi, 40 mg/kg, i.p.). Chemical analysis of this antidepressant extract allowed the isolation of (+)-Piperitol-4-O-xylopyranosyl-(1-->6)-O-glucopyranoside. This new furofuran lignan diglycoside was named tenuifloroside (1) and its complete chemical structure elucidation on the basis of 1D and 2D NMR spectra analysis of the natural compound 1 and its peracetylated derivative 1a is described. This compound was found together with two flavones-apigenin and luteolin 5-methyl ether-a phenylethanoid-verbascoside-and three iridoids-geniposide, caryoptoside and aucubin. All these compounds were purified by successive normal and reverse phase column chromatography. Tenuifloroside, caryoptoside and luteolin 5-methyl ether were isolated from Castilleja genus for the first time. These findings demonstrate that C. tenuiflora methanol extract has beneficial effect on depressive behaviors, and the knowledge of its chemical constitution allows us to propose a new standardized treatment for future investigations of this species in depressive illness.
Anti-inflammatory, antinociceptive activity of an essential oil recipe consisting of the supercritical fluid CO2 extract of white pepper, long pepper, cinnamon, saffron and myrrh in vivo.[Pubmed:25263165]
J Oleo Sci. 2014;63(12):1251-60. Epub 2014 Sep 27.
This study was designed to investigate the anti-inflammatory and antinociceptive activities of essential oil recipe (OR) in rodents. The anti-inflammatory activity was evaluated by inflammatory models of dimethylbenzene (DMB)-induced ear vasodilatation and acetic acid-induced capillary permeability enhancement in mice whereas the antinociceptive activity was evaluated using acetic acid-induced writhes and hot plate test methods in mice. Additionally, the chemical composition of OR has been also analyzed by gas chromatography and mass spectrometry (GC/MS). 37 compounds, representing 74.42% of the total oil content, were identified. beta-Selinene (7.38%), aromadendrene (5.30%), beta-elemene (5.22%), cis-Piperitol (5.21%), cis-beta-guaiene (4.67%), ylangene (3.70%), 3-heptadecene (3.55%), delta-cadinene (3%) and beta-cadinene (2.87%) were found to be the major constituents of the oil. Oral pretreatment with OR (62.5-1000 mg/kg) not only decreased the DMB-induced ear vasodilatation but also attenuated capillary permeability under acetic acid challenge in mice. OR significantly reduced the writhing number evoked by acetic acid injection. All test samples showed no significant analgesic activity on the hot plate pain threshold in mice. These data demonstrated that the OR inhibits inflammatory and peripheral inflammatory pain. These results may support the fact that the essential oil of traditional Hui prescription played a role in the inflammation of stroke.
Is differential use of Juniperus monosperma by small ruminants driven by terpenoid concentration?[Pubmed:24532215]
J Chem Ecol. 2014 Mar;40(3):285-93.
Differential plant use by herbivores has been observed for several woody plant species and has frequently been attributed to plant secondary metabolites. We examined the relationship between terpenoid concentration and Juniperus monosperma herbivory by small ruminants. Two groups of animals (10 goats or 5 goats plus 4 sheep) browsed 16 paddocks (20 x 30 m) containing one-seed juniper for six days during two seasons. Juniper leaves were sampled from 311 saplings immediately after browsing. Saplings were categorized by size (short [<0.5 m], medium [0.5-1.0 m], or tall [>1.0 m]), and by browsing intensity (light [<33 %], moderate [33-66 %], or heavy [>66 %]). Juniper bark was collected from 12 saplings during spring. Total estimated terpenoid concentrations in leaves and bark were 18.3 +/- 0.3 and 8.9 +/- 0.8 mg/g, respectively, and the dominant terpene in both tissues was alpha-pinene (11.1 +/- 0.2 and 7.6 +/- 0.7 mg/g, respectively). Total terpenoid concentration of juniper leaves was greater in spring than summer (20.6 +/- 0.5 vs. 16.7 +/- 0.3 mg/g, respectively) and was lower in short saplings than medium or tall saplings (16.5 +/- 0.6 vs. 19.8 +/- 0.4 and 19.5 +/- 0.4 mg/g, respectively). Total terpenoid concentration of leaves also differed among the three defoliation categories (21.2 +/- 0.6, 18.7 +/- 0.5, and 16.1 +/- 0.4 mg/g for light, moderate, and heavy, respectively). The smallest subset of terpenoids able to discriminate between light and heavy browsing intensity categories included eight compounds ([E]-beta-farnesene, bornyl acetate, gamma-eudesmol, endo-fenchyl acetate, gamma-cadinene, alpha-pinene, cis-Piperitol, and cis-p-menth-2-en-1-ol). Our results suggest terpenoid concentrations in one-seed juniper are related to season, sapling size, and browsing by small ruminants.
Structure-related antifeedant activity of halolactones with a p-menthane system against the lesser mealworm, Alphitobius diaperinus Panzer.[Pubmed:24009153]
Pest Manag Sci. 2014 Jun;70(6):953-8.
BACKGROUND: Feeding deterrent activity of synthetic halogen lactones against larvae and adults of the lesser mealworm, Alphitobius diaperinus Panzer, in laboratory choice and no-choice tests was studied. The compounds were synthesised from racemic and enantiomerically enriched (ee = 91-98%) cis- and trans-Piperitols, which were obtained from (+/-)-piperitone. RESULTS: Structure-activity relationship studies identified several synthetic halolactones with a very strong feeding deterrent activity. The most active were the enantiomeric chlorolactones with chiral centre configurations (1S,4S,5R,6R) and (1R,4R,5S,6S) and their racemic mixture. The racemic bromo- and iodolactones obtained from cis-Piperitol and saturated lactones with a chiral centre configuration (1R,4S,6R) were also very good antifeedants in comparison with piperitone. Most of the studied compounds were better antifeedants against adults than against larvae-among the 21 compounds, only one bromolactone with a chiral centre configuration (1S,4R,5R,6R) was a weaker deterrent for adults. CONCLUSION: Chemical transformation of the piperitone molecule by the introduction of a lactone function and a halogen atom strongly changed its antifeedant properties against the lesser mealworm. Optimum activity is dependent on the presence of a chlorine atom at C-5 of the cyclohexane ring. The activity of bromo- and iodolactones depended strongly on the chiral centre configuration and the halogen substituent.
An expedient approach to the total synthesis of (+)-5-epi-eudesm-4(15)-ene-1beta,6beta-diol.[Pubmed:23064601]
Chem Commun (Camb). 2012 Nov 25;48(91):11241-3.
The first total synthesis of (+)-5-epi-eudesm-4(15)-ene-1beta,6beta-diol has been achieved in 12 steps starting from the known (-)-cis-Piperitol and by using a chelation controlled glycolate enolate Ireland-Claisen rearrangement and an intramolecular nitrile oxide dipolar cycloaddition as key steps.
Comparative study of volatile oil compositions of two Plectranthus species from northern India.[Pubmed:21707228]
Nat Prod Res. 2011 Oct;25(18):1727-32.
The leaf and inflorescence essential oils of Plectranthus rugosus Wall. (syn. Rabdosia rugosa Wall.) and Plectranthus incanus L. (syn. Plectranthus mollis L.), which grow wild in Uttarakhand, India, were analysed and compared by capillary gas chromatography and gas chromatography-mass spectrometry. The analysis led to the identification of 43 constituents, forming 89.5-93.6% of the total oil compositions. Both leaf and inflorescence oil of P. rugosus were dominated by sesquiterpene hydrocarbons (71.8%, 71.7%) represented by beta-caryophyllene (36.2%, 29.8%), germacrene D (25.2%, 28.2%) and alpha-humulene (6.6%, 8.6%) as the major constituents. Conversely, the leaf and inflorescence oil of P. incanus were dominated by monoterpenoids (74.4%, 65.8%) with piperitenone oxide (44.2%, 38.5%), piperitone (8.6%, 12.2%) and terpinolene (14.5%, 10.2%) as major constituents. Piperitenone oxide, piperitone, cis- and trans-Piperitols and trans-Piperitol acetate were the marker constituents in P. incanus, which were not noted in the essential oil of P. rugosus.
Identification of methanol-soluble compounds in sesame and evaluation of antioxidant potential of its lignans.[Pubmed:21391595]
J Agric Food Chem. 2011 Apr 13;59(7):3214-9.
The methanol extract of sesame (Sesamum indicum) seeds was fractionated and purified with the assistance of conventional column chromatography to afford 29 compounds including seven furofuran lignans. Among these isolates, (+)-samin (1) was obtained from the natural source for the first time. In addition, (-)-asarinin (30) and sesamol (31) were generated by oxidative derivation from (+)-sesamolin (2) and (+)-sesamin (3), two abundant lignans found in sesame seeds. To evaluate their in vitro antioxidant potential, the seven isolated lignans (1-7) and the two derivatives (30 and 31) were examined for the scavenging activities on DPPH free radicals and superoxide anions. Moreover, the capability of chelating ferrous ions and reducing power of these sesame lignans were also measured. The results suggest that, besides the well-known sesamolin and sesamin, the minor sesame lignans (+)-(7S,8'R,8R)-acuminatolide (5), (-)-Piperitol (6), and (+)-pinoresinol (7) are also adequate active ingredients and may be potential sources for nutritional and pharmacological utilization.
Quantitative determination of 10 phenylpropanoid and lignan compounds in Lancea tibetica by high-performance liquid chromatography with UV detection.[Pubmed:21347996]
Planta Med. 2011 Sep;77(13):1562-6.
An HPLC method was developed for simultaneous determination of one phenylpropanoid glycoside, verbascoside (1), and nine lignans, including lantibeside (2), phillyrin (3), lantibeside B (4), lantibeside C (5), tibeticoside A (6), styraxjaponoside C (7), sylvatesmin (8), (+)-Piperitol (9), and horsfieldin (10), from the Tibetan medicinal plant Lancea tibetica Hook. F. et Thoms. The analysis was performed within 45 min. The extraction method was optimized with different solvent systems. The HPLC method was validated for linearity, repeatability, accuracy, limits of detection, and limits of quantification. The limits of detection and limits of quantification of 10 analytes were found to be less than 0.1 and 0.5 microg/mL, respectively. The RSD for intra- and inter-day analyses was less than 4.2 %, and the recovery efficiency was 90-105 %. The method was used to analyze different populations of L. tibetica collected in China. HPLC profiles showed that the concentrations of analytes were different in samples collected from different areas of China. Verbascoside was the dominant component in three out of five plant samples; compounds 2, 3, 6, and 8 accounted for over 62 % yields in total lignan contents. The method is useful for identification, quality assurance, and quality control of L. tibetica and its related products.
Secondary metabolites and cytotoxic activities from the stem bark of Zanthoxylum nitidum.[Pubmed:19551734]
Chem Biodivers. 2009 Jun;6(6):846-57.
A dihydrobenzo[c]phenanthridine alkaloid, epizanthocadinanine A (1), together with 27 known compounds, including eight benzo[c]phenanthridines, i.e., oxynitidine (2), oxyavicine (3), oxychelerythrine (4), dihydrochelerythrine (5), 6-acetonyldihydrochelerythrine (6), norchelerythrine (7), decarine (8), and arnottianamide (9); two 2-quinolones, i.e., flindersine (10) and 4-methoxy-1-methyl-2-quinolone (11); two furoquinolines, i.e., skimmianine (12) and gamma-fagarine (13); three aporphines, i.e., liriodenine (14), N-acetyldehydroanonaine (15), and N-acetylanonaine (16); six lignans, i.e., sesamin (17), episesamin (18), Piperitol-3,3-dimethylallyl ether (19), xanthoxylol-3,3-dimethylallyl ether (20), savinin (21), and 2,3-bis(3,4-methylenedioxybenzyl)but-2-en-4-olide (22); three terpenoids, i.e., alpha-cadinol (23), anticopalol (24), and spathulenol (25); one coumarin, i.e., aesculetin dimethyl ether (26); and two steroids, i.e., beta-sitosterol (27) and beta-sitostenone (28) were isolated from the stem bark of Zanthoxylum nitidum. Their structures were elucidated on the basis of extensive 1D- and 2D-NMR as well as MS analyses. Moreover, the recently reported structures 2'-4' of rhoifolines B and A, and '8-methoxynorchelerythrine', resp., isolated as new compounds from Z. rhoifolium and Z. nitidum, resp., could be assigned the revised structures 2-4 by reinvestigation of the spectroscopic data. In addition, the cytotoxicity of the isolates was evaluated on the MCF-7, NCI-H460, and SF-268 cell lines. Among these isolates, liriodenine (14) was the most active compound against the MCF-7, NCI-H460, and SF-268 cell lines with IC(50) values of 2.19, 2.38, and 3.19 microg/ml, resp.
Lignan derivatives from the stem bark of Syzygium cumini (L.) Skeels.[Pubmed:19296384]
Nat Prod Res. 2009;23(5):422-30.
Phytochemical investigation of the stem bark of Syzygium cumini (L.) Skeels (Myrtaceae) yielded four new lignan derivatives characterised as (7alpha,8alpha,2'alpha)-3,4,5-trimethoxy-7,3',1',9'-diepoxylignan (cuminiresinol), (7alpha,7'alpha,8alpha,8'alpha)-3,4-dioxymethylene-3',4'-dimethoxy-7,9',7',9-diep oxylignan-5'-ol (5'-hydroxy-methyl-Piperitol), (7alpha,7'alpha,8alpha,8'alpha)-3'-methoxy-9-oxo-7,9',7',9-diepoxylignan-3,4,4'-t riol or 3-demethyl-9-oxo-pinoresinol (syzygiresinol A), (7alpha,7'alpha,8alpha,8'alpha)-9-oxo-7,9',7',9-diepoxylignan-3,4,3',4',5'-pentao l or 3,3'-didemethyl-9-oxo-pinoresinol (syzygiresinol B) along with the known lignans di-demethyl-5-hydroxypinoresinol, dimethylpinoresinol, didemethoxypinoresinol, pinoresinol and 4'-methyl-5'-hydroxypinoresinol. The structures of these lignans were elucidated on the basis of structural data analysis and chemical reactions.
Composition and antifungal activity of the essential oil of the Brazilian Chenopodium ambrosioides L.[Pubmed:18679750]
J Chem Ecol. 2008 Sep;34(9):1213-8.
The antifungal activity of essential oil (EO) from the Brazilian epazote (Chenopodium ambrosioides L.) was evaluated by the poison food assay at concentrations of 0.3%, 0.1%, and 0.05% with eight postharvest deteriorating fungi (Aspergillus flavus, Aspergillus glaucus, Aspergillus niger, Aspergillus ochraceous, Colletotrichum gloesporioides, Colletotrichum musae, Fusarium oxysporum, and Fusarium semitectum). EO components were tentatively identified by Kovats retention indices (RIs) using gas chromatography and gas chromatography combined with mass spectrometry (GC-MS). Growth of all fungi was completely inhibited at 0.3% concentration, and by 90% to 100% at 0.1% concentration. The following 13 tentatively identified compounds (relative percent) accounted for 90.4% of the total volatile oil: alpha-terpinene (0.9), p-cymene (2.0), benzyl alcohol (0.3), p-cresol (0.3), p-mentha-1,3,8-triene (0.2), p-cimen-8-ol (0.6), alpha-terpineol (0.5), (Z)-ascaridole (61.4), piperitone (0.9), carvacrol (3.9), (E)-ascaridole (18.6), (E)-Piperitol acetate (0.5), and (Z)-carvyl acetate (0.3). Autobiographic thin layer chromatography of the EO to separate the principal fungitoxic fraction yielded only one fraction that completely inhibited the growth of all test fungi at a concentration of 0.1%. This fraction was characterized by RIs and GC-MS presenting a composition (%) of p-cymene (25.4), (Z)-ascaridole (44.4), and (E)-ascaridole (30.2). The results suggest ascaridoles were the principal fungitoxic components of the EO.
Hinokinin biosynthesis in Linum corymbulosum Reichenb.[Pubmed:18489708]
Plant J. 2008 Sep;55(5):810-20.
SUMMARY: Due to their peculiar stereochemistry and numerous biological activities, lignans are of widespread interest. As only a few biosynthetic steps have been clarified to date, we aimed to further resolve the molecular basis of lignan biosynthesis. To this end, we first established that the biologically active lignan (-)-hinokinin could be isolated from in vitro cultures of Linum corymbulosum. Two hypothetical pathways were outlined for the biosynthesis of (-)-hinokinin. In both pathways, (+)-pinoresinol serves as the primary substrate. In the first pathway, pinoresinol is reduced via lariciresinol to secoisolariciresinol by a pinoresinol-lariciresinol reductase, and methylenedioxy bridges are formed later. In the second pathway, pinoresinol itself is the substrate for formation of the methylenedioxy bridges, resulting in consecutive production of Piperitol and sesamin. To determine which of the proposed hypothetical pathways acts in vivo, we first isolated several cDNAs encoding one pinoresinol-lariciresinol reductase (PLR-Lc1), two phenylcoumaran benzylic ether reductases (PCBER-Lc1 and PCBER-Lc2), and two PCBER-like proteins from a cDNA library of L. corymbulosum. PLR-Lc1 was found to be enantiospecific for the conversion of (+)-pinoresinol to (-)-secoisolariciresinol, which can be further converted to give (-)-hinokinin. Hairy root lines with significantly reduced expression levels of the plr-Lc1 gene were established using RNAi technology. Hinokinin accumulation was reduced to non-detectable levels in these lines. Our results strongly indicate that PLR-Lc1 participates in (-)-hinokinin biosynthesis in L. corymbulosum by the first of the two hypothetical pathways via (-)-secoisolariciresinol.
Anti-inflammatory and analgesic activities of the ethanolic extracts from Zanthoxylum riedelianum (Rutaceae) leaves and stem bark.[Pubmed:17725859]
J Pharm Pharmacol. 2007 Aug;59(8):1151-8.
We have evaluated the anti-inflammatory and analgesic properties of the leaves (LCE) and stem bark (BCE) crude extracts of Zanthoxylum riedelianum (Rutaceae). Different fractions of the stem bark extract (hexane, BCEH; dichloromethane, BCED; ethyl acetate, BCEE; and lyophilized aqueous residual, BCEW) were also investigated. We studied the effects of the extracts and fractions using the rat paw oedema test induced by carrageenan, dextran, histamine or nystatin; the mouse abdominal constriction test; the mouse hot-plate test (only for LCE and BCE); and the mouse formalin test. Both extracts and all BCE fractions displayed anti-inflammatory activity in the carrageenan-induced oedema model, but not for dextran, histamine or nystatin. Considering the analgesic models, both extracts showed antinociceptive activity, but BCE was more active than LCE in models of central pain. All BCE fractions showed significant inhibition in the abdominal constriction test and in both phases of the formalin test. When BCED was submitted to phytochemical procedures it led to the isolation of six lignans (sesamin, methylpluviatolide, dimethylmatairesinol, Piperitol-4(')-O-(gamma),(gamma)-dimethylallyl ether, kaerophyllin and hinokinin), and a triterpene (lupeol). Inhibition of cyclooxygenase and its metabolites may have been involved in the mechanism of action of this plant, considering previous studies reporting the anti-inflammatory and analgesic activity for the identified lignans, as well as anti-inflammatory activity for lupeol.


