PamabromDiuretic for relieving menstrual-associated symptoms CAS# 606-04-2 |
- GDC-0068 (RG7440)
Catalog No.:BCC1271
CAS No.:1001264-89-6
- MK-2206 dihydrochloride
Catalog No.:BCC1274
CAS No.:1032350-13-2
- AZD5363
Catalog No.:BCC1073
CAS No.:1143532-39-1
- A-443654
Catalog No.:BCC1321
CAS No.:552325-16-3
- AKT inhibitor VIII
Catalog No.:BCC1334
CAS No.:612847-09-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 606-04-2 | SDF | Download SDF |
| PubChem ID | 11806 | Appearance | Powder |
| Formula | C11H18BrN5O3 | M.Wt | 348.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 150 mg/mL (430.79 mM; Need ultrasonic) | ||
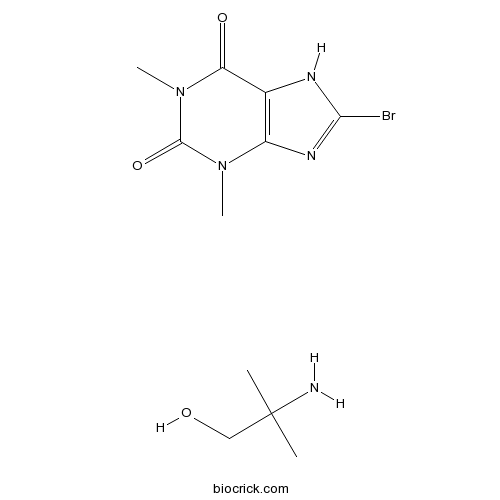
| Chemical Name | 2-amino-2-methylpropan-1-ol;8-bromo-1,3-dimethyl-7H-purine-2,6-dione | ||
| SMILES | CC(C)(CO)N.CN1C2=C(C(=O)N(C1=O)C)NC(=N2)Br | ||
| Standard InChIKey | ATOTUUBRFJHZQG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H7BrN4O2.C4H11NO/c1-11-4-3(9-6(8)10-4)5(13)12(2)7(11)14;1-4(2,5)3-6/h1-2H3,(H,9,10);6H,3,5H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pamabron is a common over-the-counter diuretic used for relief of menstrual-associated symptoms. The active diuretic ingredient in pamabrom is 8-bromotheophylline. Pamabrom is available in combination with acetaminophen (paracetamol) for various conditions such as back pain and menstrual relief. The acetaminophen helps reduce menstrual pains and the pamabrom reduces associated bloating. References: | |||||

Pamabrom Dilution Calculator

Pamabrom Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8719 mL | 14.3596 mL | 28.7191 mL | 57.4383 mL | 71.7978 mL |
| 5 mM | 0.5744 mL | 2.8719 mL | 5.7438 mL | 11.4877 mL | 14.3596 mL |
| 10 mM | 0.2872 mL | 1.436 mL | 2.8719 mL | 5.7438 mL | 7.1798 mL |
| 50 mM | 0.0574 mL | 0.2872 mL | 0.5744 mL | 1.1488 mL | 1.436 mL |
| 100 mM | 0.0287 mL | 0.1436 mL | 0.2872 mL | 0.5744 mL | 0.718 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pamabron is a common over-the-counter diuretic used for relief of menstrual-associated symptoms. The active diuretic ingredient in pamabrom is 8-bromotheophylline. Pamabrom is available in combination with acetaminophen (paracetamol) for various conditions such as back pain and menstrual relief. The acetaminophen helps reduce menstrual pains and the pamabrom reduces associated bloating.
- Tirandamycin B
Catalog No.:BCN1862
CAS No.:60587-14-6
- DCEBIO
Catalog No.:BCC7060
CAS No.:60563-36-2
- P1075
Catalog No.:BCC7027
CAS No.:60559-98-0
- 1-Acetyltagitinin A
Catalog No.:BCN4119
CAS No.:60547-63-9
- Gomisin D
Catalog No.:BCN2268
CAS No.:60546-10-3
- Olsalazine Sodium
Catalog No.:BCC3829
CAS No.:6054-98-4
- Braylin
Catalog No.:BCN4118
CAS No.:6054-10-0
- Methyloleoside
Catalog No.:BCN8079
CAS No.:60539-23-3
- H-D-Thr-OMe.HCl
Catalog No.:BCC2675
CAS No.:60538-15-0
- Zimelidine dihydrochloride
Catalog No.:BCC7173
CAS No.:60525-15-7
- 5,6-Dihydropyridin-2(1H)-one
Catalog No.:BCN4013
CAS No.:6052-73-9
- Serpentinic acid
Catalog No.:BCN4616
CAS No.:605-14-1
- 2,4'-Dihydroxybenzophenone
Catalog No.:BCN3358
CAS No.:606-12-2
- Toyocamycin
Catalog No.:BCC8047
CAS No.:606-58-6
- Cinnabarinic acid
Catalog No.:BCC7865
CAS No.:606-59-7
- Homopterocarpin
Catalog No.:BCN4615
CAS No.:606-91-7
- MK-0773
Catalog No.:BCC1754
CAS No.:606101-58-0
- Momor-cerebroside I
Catalog No.:BCN4120
CAS No.:606125-07-9
- AZD6244 (Selumetinib)
Catalog No.:BCC3624
CAS No.:606143-52-6
- MEK162 (ARRY-162, ARRY-438162)
Catalog No.:BCC1148
CAS No.:606143-89-9
- Bifonazole
Catalog No.:BCC4766
CAS No.:60628-96-8
- Danaidone
Catalog No.:BCN1966
CAS No.:6064-85-3
- Dihydromollugin
Catalog No.:BCN8247
CAS No.:60657-93-4
- 3-n-Butylphthalide
Catalog No.:BCN2381
CAS No.:6066-49-5
Erythema multiforme secondary to dimenhydrinate in a patient with previous similar reactions to pamabrom.[Pubmed:24396093]
Ann Pharmacother. 2014 Mar;48(3):425-8.
OBJECTIVE: To report a case of erythema multiforme secondary to dimenhydrinate and Pamabrom cross-sensitivity. CASE SUMMARY: A 22-year-old Chinese female presented with a complaint of lip mucosal ulceration with necrosis and stomatitis, worsening over the past 24 hours and associated with reduced oral intake and incomplete opening of the mouth. Presentation was accompanied by a generalized rash and genital mucosal involvement. The only new systemically ingested agent was dimenhydrinate approximately 4 days prior to admission. She had no significant medical history, but was labeled to be allergic to acetaminophen. She had a positive history of 2 similar presentations secondary to Panadol Menstrual (acetaminophen and Pamabrom), once 3 years ago and again 5 months prior to the current admission. An objective causality assessment revealed that the adverse drug event was "probable" to dimenhydrinate. A detailed history revealed a negative drug challenge to acetaminophen. She had previously taken plain acetaminophen and Beserol (acetaminophen and chlormezanone) with no reaction. DISCUSSION: A comprehensive history taking facilitated the diagnosis of erythema multiforme secondary to dimenhydrinate without the need to perform invasive testing, and the removal of erroneous allergy labeling to acetaminophen. Dimenhydrinate and pamabron both contain theophylline-related structures in their chemical composition. Similar reactions to Pamabrom strongly suggested cross-sensitivity to theophylline-related structures. CONCLUSIONS: To our knowledge, this is the first report of erythema multiforme due to dimenhydrinate with pamabron cross-sensitivity. We recommend that comprehensive medication-history taking be carried out for all drug-allergy patients to ensure greater informed decision making when choosing medications to use for that patient in the future.
Development and Validation of Stability-indicating RP-HPLC Method for Estimation of Pamabrom in Tablets.[Pubmed:25035530]
Indian J Pharm Sci. 2014 May;76(3):198-202.
The present study depicts the development of a validated RP-HPLC method for the determination of the Pamabrom in presence of degradation products or other pharmaceutical excipients. Stress study was performed on Pamabrom and it was found that it degrade sufficiently in acidic, alkali and oxidative condition but less degradation was found in thermal and photolytic condition. The separation was carried out on Enable G 120 A(0) (250x4.6 mm, 5 mu) column having particle size 5 mu using methanol: water (75:25 v/v) with pH 4.0 adjusted with ortho phosphoric acid as mobile phase at flow rate of 1 ml/min. The wavelength of the detection was 280nm. A retention time (Rt) nearly 3.9 min was observed. The calibration curve for Pamabrom was linear (r (2) = 0.9997) from range of 10-60 mug/ml with limit of detection and limit of quantification of 1.41 mug/ml and 4.28 mug/ml, respectively. Analytical validation parameter such as selectivity, specificity, linearity, accuracy and precision were evaluated and relative standard deviation value for all the key parameters were less than 2.0%. The recovery of the drug after standard addition was found to be 101.35%. Thus, the developed RP-HPLC method was found to be suitable for the determination of Pamabrom in bulk as well as stability samples of tablets containing various excipients.
Naproxen, paracetamol and pamabrom versus paracetamol, pyrilamine and pamabrom in primary dysmenorrhea: a randomized, double-blind clinical trial.[Pubmed:27813503]
Medwave. 2016 Oct 24;16(9):e6587.
INTRODUCTION: Dysmenorrhea is caused by the discharge of prostaglandins into the uterine tissue; therefore, non-steroidal anti-inflammatory drugs (NSAIDs) are the established initial therapy for dysmenorrhea. Dysmenorrhea therapy may include the administration of drug monotherapy or combination therapy. However, clinical scientific evidence on the efficacy of medications with two or three drugs combined is scarce or nonexistent. OBJECTIVE: To evaluate and compare the efficacy and safety of two oral fixed-dose combinations for the relief of the symptoms of primary dysmenorrhea among Mexican women. One of the combinations is widely used in Mexico (paracetamol, pyrilamine and Pamabrom) and the selected comparison was a medication with naproxen sodium, paracetamol and Pamabrom based on the pathophysiology of primary dysmenorrhea. METHODS: This was a single-centre, double blind, experimental, parallel group, randomized trial. Female patients with primary dysmenorrhea, older than 17 years and with pain intensity greater than 45 mm on a visual analogue scale, were included. The patients were then randomized to receive tablets with naproxen sodium, paracetamol and Pamabrom or tablets with paracetamol, pyrilamine and Pamabrom for one menstrual cycle. Patient evaluations of symptomatology and pain intensity were recorded throughout one menstrual period. Descriptive and inferential statistical analyses were utilized. RESULTS: An intention-to-treat population of 91 women, with a mean age of 21.3 +/- 3.2 years, received paracetamol, pyrilamine and Pamabrom tablets, and 98 participants, with a mean age of 21.0 +/- 3.2 years, received naproxen sodium, paracetamol and Pamabrom tablets. The participants assessments of pain on the Visual Analogue Scale during the menstrual cycle demonstrated a significant reduction in both treatment groups (p<0.05). There is no significant difference in efficacy between both groups (p>0.05). CONCLUSIONS: The results showed that both drug combinations were not different in reducing dysmenorrheic pain. Likewise, both treatments were well tolerated. Therefore, both treatments may be used for the treatment of primary dysmenorrhea.
HPTLC and RP-HPLC methods for simultaneous determination of Paracetamol and Pamabrom in presence of their potential impurities.[Pubmed:26001162]
J Pharm Biomed Anal. 2015 Oct 10;114:22-7.
Two chromatgraphic methods were developed for determination of Paracetamol (PCM) and Pamabrom (PAM) in presence of P-aminophenol (PAP) and Theophylline (THEO) as potential impurities of both drugs respectively. First method is HPTLC which depends on separation and quantitation of the studied drugs on aluminum plates pre-coated with silica gel 60 F(2)(5)(4) as a stationary phase using chloroform:methanol:ethyl acetate:glacial acetic acid (8:0.8:0.6:0.2, v/v/v/v) as mobile phase followed by densitometric measurement of the bands at 254 nm. Second method is RP-HPLC which comprises separation of the studied drugs on a Phenomenex C8 column by gradient elution using mobile phase consisting of sodium dihydrogen phosphate buffer (0.05 M): methanol:acetonitrile (85:10:5, v/v/v) at a flow rate of 1 mL/min for first 7.5 min and (70:20:10, v/v/v) at a flow rate of 1.5 mL/min for the next 5 min. The proposed methods were successfully applied for determination of the potential impurities of PCM and PAM after resolving them from the pure drugs. The developed methods have been validated and proved to meet the requirements delineated by ICH guidelines with respect to linearity, accuracy, precision, specificity and robustness. The validated methods were successfully applied for determination of the studied drugs in their pharmaceutical formulation. The results were statistically compared to those obtained by the reported RP-HPLC method where no significant difference was found; indicating the ability of proposed methods to be used for routine quality control analysis of these drugs.


