Cinnabarinic acidSelective mGlu4 agonist CAS# 606-59-7 |
- CFM 1571 hydrochloride
Catalog No.:BCC5924
CAS No.:1215548-30-3
- A 350619 hydrochloride
Catalog No.:BCC5939
CAS No.:1217201-17-6
- BAY 41-2272
Catalog No.:BCC7932
CAS No.:256376-24-6
- Riociguat
Catalog No.:BCC1899
CAS No.:625115-55-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 606-59-7 | SDF | Download SDF |
| PubChem ID | 114918 | Appearance | Powder |
| Formula | C14H8N2O6 | M.Wt | 300.22 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
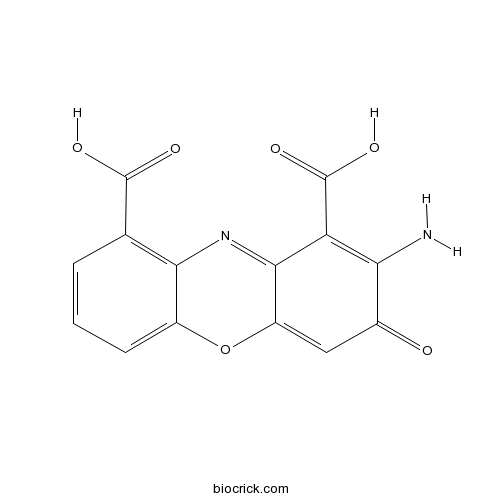
| Chemical Name | 2-amino-3-oxophenoxazine-1,9-dicarboxylic acid | ||
| SMILES | C1=CC(=C2C(=C1)OC3=CC(=O)C(=C(C3=N2)C(=O)O)N)C(=O)O | ||
| Standard InChIKey | FSBKJYLVDRVPTK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H8N2O6/c15-10-6(17)4-8-12(9(10)14(20)21)16-11-5(13(18)19)2-1-3-7(11)22-8/h1-4H,15H2,(H,18,19)(H,20,21) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | mGlu4 receptor agonist. Activates mGlu4 receptors in transiently transfected HEK293 cells; selective over all other mGlu subtypes (mGlu1, mGlu2 and mGlu5-8). Decreases cAMP levels in native cultured cerebellar granule cells. Also counteracts excitotoxic neuronal cell death induced by NMDA in mixed cultures of cortical cells; displays neuroprotective activity. |

Cinnabarinic acid Dilution Calculator

Cinnabarinic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3309 mL | 16.6545 mL | 33.3089 mL | 66.6178 mL | 83.2723 mL |
| 5 mM | 0.6662 mL | 3.3309 mL | 6.6618 mL | 13.3236 mL | 16.6545 mL |
| 10 mM | 0.3331 mL | 1.6654 mL | 3.3309 mL | 6.6618 mL | 8.3272 mL |
| 50 mM | 0.0666 mL | 0.3331 mL | 0.6662 mL | 1.3324 mL | 1.6654 mL |
| 100 mM | 0.0333 mL | 0.1665 mL | 0.3331 mL | 0.6662 mL | 0.8327 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Toyocamycin
Catalog No.:BCC8047
CAS No.:606-58-6
- 2,4'-Dihydroxybenzophenone
Catalog No.:BCN3358
CAS No.:606-12-2
- Pamabrom
Catalog No.:BCC1835
CAS No.:606-04-2
- Tirandamycin B
Catalog No.:BCN1862
CAS No.:60587-14-6
- DCEBIO
Catalog No.:BCC7060
CAS No.:60563-36-2
- P1075
Catalog No.:BCC7027
CAS No.:60559-98-0
- 1-Acetyltagitinin A
Catalog No.:BCN4119
CAS No.:60547-63-9
- Gomisin D
Catalog No.:BCN2268
CAS No.:60546-10-3
- Olsalazine Sodium
Catalog No.:BCC3829
CAS No.:6054-98-4
- Braylin
Catalog No.:BCN4118
CAS No.:6054-10-0
- Methyloleoside
Catalog No.:BCN8079
CAS No.:60539-23-3
- H-D-Thr-OMe.HCl
Catalog No.:BCC2675
CAS No.:60538-15-0
- Homopterocarpin
Catalog No.:BCN4615
CAS No.:606-91-7
- MK-0773
Catalog No.:BCC1754
CAS No.:606101-58-0
- Momor-cerebroside I
Catalog No.:BCN4120
CAS No.:606125-07-9
- AZD6244 (Selumetinib)
Catalog No.:BCC3624
CAS No.:606143-52-6
- MEK162 (ARRY-162, ARRY-438162)
Catalog No.:BCC1148
CAS No.:606143-89-9
- Bifonazole
Catalog No.:BCC4766
CAS No.:60628-96-8
- Danaidone
Catalog No.:BCN1966
CAS No.:6064-85-3
- Dihydromollugin
Catalog No.:BCN8247
CAS No.:60657-93-4
- 3-n-Butylphthalide
Catalog No.:BCN2381
CAS No.:6066-49-5
- HOSu
Catalog No.:BCC2845
CAS No.:6066-82-6
- Isopicropodophyllone
Catalog No.:BCN8316
CAS No.:60660-50-6
- 3-Hydroxy-1,5-diphenyl-1-pentanone
Catalog No.:BCN3536
CAS No.:60669-64-9
Cinnabarinic acid and xanthurenic acid: Two kynurenine metabolites that interact with metabotropic glutamate receptors.[Pubmed:27342123]
Neuropharmacology. 2017 Jan;112(Pt B):365-372.
Cinnabarinic and xanthurenic acids are kynurenine metabolites generated by oxidative dimerization of 3-hydroxyanthranilic acid and transamination of 3-hydroxykynurenine, respectively. Recent evidence suggests that both compounds can affect brain function and neurotransmission and interact with metabotropic glutamate (mGlu) receptors. Cinnabarinic acid behaves as an orthosteric agonist of mGlu4 receptors, whereas some of the in vitro and in vivo effects produced by xanthurenic acid appear to be mediated by the activation of mGlu2 and mGlu3 receptors. Cinnabarinic acid could play an important role in mechanisms of neuroinflammation acting as a linking bridge between the immune system and the CNS. Xanthurenic acid has potential implications in the pathophysiology of schizophrenia and is a promising candidate as a peripheral biomarker of the disorder. The action of Cinnabarinic acid and xanthurenic acid may extend beyond the regulation of mGlu receptors and may involve several diverse molecular targets, such as the aryl hydrocarbon receptor for Cinnabarinic acid and vesicular glutamate transporters for xanthurenic acid. The growing interest on these two metabolites of the kynurenine pathway may unravel new aspects in the complex interaction between tryptophan metabolism and brain function, and lead to the discovery of new potential targets for the treatment of neurological and psychiatric disorders. This article is part of the Special Issue entitled 'The Kynurenine Pathway in Health and Disease'.
Adsorption of cinnabarinic acid from culture fluid with magnetic microbeads.[Pubmed:25994378]
Biomed Chromatogr. 2016 Feb;30(2):88-96.
In this study, antimicrobial pigment Cinnabarinic acid (CA) was produced from Pycnoporus cinnabarinus in laboratory-scale batch cultures. Magnetic poly(ethylene glycol dimethacrylate-N-methacryloyl-l-tryptophan methyl ester) [m-poly(EGDMA-MATrp)] beads (average diameter = 53-103 microm) were synthesized by copolymerizing of N-methacryloyl-l-tryptophan methyl ester (MATrp) with ethylene glycol dimethacrylate (EGDMA) in the presence of magnetite (Fe3O4) and used for the adsorption of CA. The m-poly(EGDMA-MATrp) beads were characterized by N2 adsorption/desorption isotherms (Brunauer Emmet Teller), X-ray photoelecron spectroscopy, scanning electron microscopy, infrared spectroscopy, thermal gravimetric analysis, electron spin resonance and swelling studies. The efficiency of m-poly(EGDMA-MATrp) beads for separation of CA from culture fluid was evaluated. The effects of pH, initial concentration, contact time and temperature on adsorption were analyzed. The maximum CA adsorption capacity of the m-poly(EGDMA-MATrp) beads was 272.9 mg g(-1) at pH 7.0, 25 degrees C. All the isotherm data can be fitted with the Langmuir, Freundlich and Dubinin-Radushkevich isotherm models. The adsorption process obeyed pseudo-second-order kinetic model. Thermodynamic parameters DeltaH = 5.056 kJ mol(-1), DeltaS = 52.44 J K(-1) mol(-1) and DeltaG = -9.424 kJ mol-(1) to -11.27 kJ mol-(1) with the rise in temperature from 4 to 40 degrees C indicated that the adsorption process was endothermic and spontaneous.
Cinnabarinic acid, an endogenous agonist of type-4 metabotropic glutamate receptor, suppresses experimental autoimmune encephalomyelitis in mice.[Pubmed:24565643]
Neuropharmacology. 2014 Jun;81:237-43.
Cinnabarinic acid (CA) is an endogenous metabolite of the kynurenine pathway which acts as an orthosteric agonist of type-4 metabotropic glutamate receptor (mGlu4). We now report that systemic administration of CA (0.1-10 mg/kg, i.p.) was highly protective against experimental autoimmune encephalomyelitis (EAE) induced by the myelin oligodendrocyte glycoprotein (MOG35-55) peptide, which models multiple sclerosis in mice. Full protection against EAE required daily injections of CA since the time of immunization, similarly to what reported for the mGlu4 enhancer N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1acarboxamide (PHCCC). CA treatment boosted an immune response dominated by regulatory T (Treg) cells at the expenses of Th17 cells. In addition, exogenous CA enhanced endogenous CA formation in lymphocytes, suggesting the occurrence of a positive feedback loop sustaining immune tolerance. To examine whether activation of mGlu4 could account for the protective activity of CA against EAE, we used mGlu4 knockout mice. As expected, these mice displayed a more severe form of EAE in response to immunization. CA was still protective against EAE in mGlu4-deficient mice, although its action was significantly reduced both at high and low CA doses. This suggests that the action of CA against neuroinflammation involves multiple mechanisms including the activation of mGlu4. These data further suggest that CA is one possible bridge between activation of the kynurenine pathway and immune tolerance aimed at restraining neuroinflammation.
Cinnabarinic acid, an endogenous metabolite of the kynurenine pathway, activates type 4 metabotropic glutamate receptors.[Pubmed:22311707]
Mol Pharmacol. 2012 May;81(5):643-56.
Cinnabarinic acid is an endogenous metabolite of the kynurenine pathway that meets the structural requirements to interact with glutamate receptors. We found that Cinnabarinic acid acts as a partial agonist of type 4 metabotropic glutamate (mGlu4) receptors, with no activity at other mGlu receptor subtypes. We also tested the activity of Cinnabarinic acid on native mGlu4 receptors by examining 1) the inhibition of cAMP formation in cultured cerebellar granule cells; 2) protection against excitotoxic neuronal death in mixed cultures of cortical cells; and 3) protection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity in mice after local infusion into the external globus pallidus. In all these models, Cinnabarinic acid behaved similarly to conventional mGlu4 receptor agonists, and, at least in cultured neurons, the action of low concentrations of Cinnabarinic acid was largely attenuated by genetic deletion of mGlu4 receptors. However, high concentrations of Cinnabarinic acid were still active in the absence of mGlu4 receptors, suggesting that the compound may have off-target effects. Mutagenesis and molecular modeling experiments showed that Cinnabarinic acid acts as an orthosteric agonist interacting with residues of the glutamate binding pocket of mGlu4. Accordingly, Cinnabarinic acid did not activate truncated mGlu4 receptors lacking the N-terminal Venus-flytrap domain, as opposed to the mGlu4 receptor enhancer, N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide (PHCCC). Finally, we could detect endogenous Cinnabarinic acid in brain tissue and peripheral organs by high-performance liquid chromatography-tandem mass spectrometry analysis. Levels increased substantially during inflammation induced by lipopolysaccharide. We conclude that Cinnabarinic acid is a novel endogenous orthosteric agonist of mGlu4 receptors endowed with neuroprotective activity.


