PNU 109291Potent and selective 5-HT1D agonist CAS# 187665-60-7 |
- PF-562271
Catalog No.:BCC3674
CAS No.:717907-75-0
- TAE226 (NVP-TAE226)
Catalog No.:BCC3885
CAS No.:761437-28-9
- PF-573228
Catalog No.:BCC4496
CAS No.:869288-64-2
- PF-00562271
Catalog No.:BCC3684
CAS No.:939791-38-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 187665-60-7 | SDF | Download SDF |
| PubChem ID | 10621491 | Appearance | Powder |
| Formula | C24H31N3O3 | M.Wt | 409.52 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
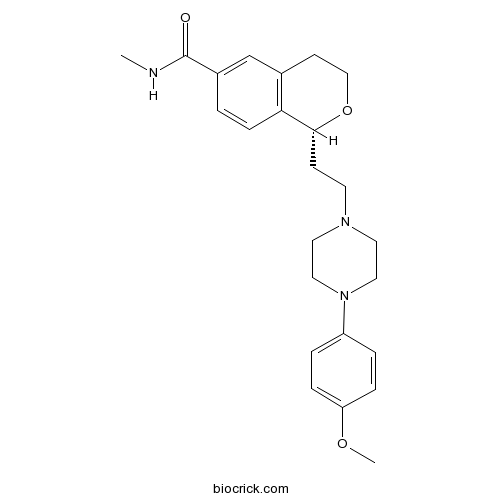
| Chemical Name | (1R)-1-[2-[4-(4-methoxyphenyl)piperazin-1-yl]ethyl]-N-methyl-3,4-dihydro-1H-isochromene-6-carboxamide | ||
| SMILES | CNC(=O)C1=CC2=C(C=C1)C(OCC2)CCN3CCN(CC3)C4=CC=C(C=C4)OC | ||
| Standard InChIKey | UDLSEQDYARNKTL-HSZRJFAPSA-N | ||
| Standard InChI | InChI=1S/C24H31N3O3/c1-25-24(28)19-3-8-22-18(17-19)10-16-30-23(22)9-11-26-12-14-27(15-13-26)20-4-6-21(29-2)7-5-20/h3-8,17,23H,9-16H2,1-2H3,(H,25,28)/t23-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective 5-HT1D receptor agonist that displays > 600-fold selectivity over 5-HT1A and 5-HT2A receptors and no activity at 5-HT1B, 5-HT1E, 5-HT2B, 5-HT2C, 5-HT6 and 5-HT7 receptors. Reduces dural plasma extravasation evoked by trigeminal ganglion stimulation. |

PNU 109291 Dilution Calculator

PNU 109291 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4419 mL | 12.2094 mL | 24.4188 mL | 48.8377 mL | 61.0471 mL |
| 5 mM | 0.4884 mL | 2.4419 mL | 4.8838 mL | 9.7675 mL | 12.2094 mL |
| 10 mM | 0.2442 mL | 1.2209 mL | 2.4419 mL | 4.8838 mL | 6.1047 mL |
| 50 mM | 0.0488 mL | 0.2442 mL | 0.4884 mL | 0.9768 mL | 1.2209 mL |
| 100 mM | 0.0244 mL | 0.1221 mL | 0.2442 mL | 0.4884 mL | 0.6105 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tataramide B
Catalog No.:BCN3897
CAS No.:187655-56-7
- Fmoc-D-Arg(Pbf)-OH
Catalog No.:BCC3077
CAS No.:187618-60-6
- Ethyl rutinoside
Catalog No.:BCC8976
CAS No.:187539-57-7
- MaxiPost
Catalog No.:BCC7984
CAS No.:187523-35-9
- N-Aminophthalimide
Catalog No.:BCC9085
CAS No.:1875-48-5
- Sitoindoside I
Catalog No.:BCN1161
CAS No.:18749-71-8
- Methylisopelletierine
Catalog No.:BCN1160
CAS No.:18747-42-7
- ER 50891
Catalog No.:BCC7783
CAS No.:187400-85-7
- Boc-D-FMK
Catalog No.:BCC1128
CAS No.:187389-53-3,634911-80-1
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Kimcuongin
Catalog No.:BCN7472
CAS No.:1872403-23-0
- PA-824
Catalog No.:BCC1106
CAS No.:187235-37-6
- PNU 142633
Catalog No.:BCC7222
CAS No.:187665-65-2
- Fmoc-Asp-OFm
Catalog No.:BCC3467
CAS No.:187671-16-5
- BIO 1211
Catalog No.:BCC3945
CAS No.:187735-94-0
- Serpentine
Catalog No.:BCN1162
CAS No.:18786-24-8
- Arachidonyl serotonin
Catalog No.:BCC7500
CAS No.:187947-37-1
- (+)-Corynoline
Catalog No.:BCN1235
CAS No.:18797-79-0
- Acetylcorynoline
Catalog No.:BCN1239
CAS No.:18797-80-3
- WKYMVM trifluoroacetate salt
Catalog No.:BCC5815
CAS No.:187986-11-4
- Trp-Lys-Tyr-Met-Val-Met
Catalog No.:BCC5816
CAS No.:187986-17-0
- 5-Acetyltaxachitriene A
Catalog No.:BCN7412
CAS No.:187988-48-3
- Boc-Glu-NH2
Catalog No.:BCC3387
CAS No.:18800-74-3
- Bikinin
Catalog No.:BCC5582
CAS No.:188011-69-0
Effects of PNU-109,291, a selective 5-HT1D receptor agonist, on electrically induced dural plasma extravasation and capsaicin-evoked c-fos immunoreactivity within trigeminal nucleus caudalis.[Pubmed:10428423]
Neuropharmacology. 1999 Jul;38(7):1043-53.
We studied the effects of PNU-109291 [(S)-(-)-1-[2-[4-(4-methoxyphenyl)-1-piperazinyl]ethyl]-N-methyl-isoc hroman-6-carboxamide], a receptor agonist showing 5000-fold selectivity for primate 5-HT1D versus 5-HT1B receptors (Ennis et al., J. Med. Chem. 41, 2180-2183), on dural neurogenic inflammation and on c-fos like immunoreactivity within trigeminal nucleus caudalis evoked by electrical and chemical activation of trigeminal afferents, respectively. Subcutaneous injection of PNU-109291 in male guinea pigs dose-dependently reduced dural extravasation of [125I]-labeled bovine serum albumin evoked by trigeminal ganglion stimulation with an IC50 of 4.2 nmol kg(-1). A dose of 73.3 nmol kg(-1) blocked the response completely. The selective 5-HT1B/1D receptor antagonist GR-127935 (> or = 2 micromol kg(-1) i.v.) prevented this effect. In addition, the number of c-fos immunoreactive cells within guinea pig trigeminal nucleus caudalis induced by chemical meningeal stimulation (intracisternally administered capsaicin) was reduced by more than 50% with PNU-109291 (> or = 122.2 nmol kg(-1) administered s.c. 45 min before and 15 min after capsaicin). These data indicate that the 5-HT1D receptor subtype plays a significant role in suppressing meningeal neurogenic inflammation and attenuating trigeminal nociception in these guinea pig models. Since 5-HT1D receptor mRNA and protein are expressed in trigeminal ganglia but not vascular smooth muscle, the 5-HT1D receptor subtype may become a useful therapeutic target for migraine and related headaches.
5-Hydroxytryptamine1B receptor-mediated contraction of rabbit saphenous vein and basilar artery: role of vascular endothelium.[Pubmed:14724223]
J Pharmacol Exp Ther. 2004 May;309(2):825-32.
This study characterizes the sumatriptan-sensitive [5-hydroxytryptamine (5-HT)(1B/1D)] receptor in rabbit saphenous vein and basilar artery. (S)-(-)-1-[2-[4-(4-Methoxy-phenyl)-piperazin-1-yl]-ethyl]isochroman-6-carboxylic acid methylamide (PNU-109291), a 5-HT(1D) subtype-selective agonist (human K(i) = 2.5 +/- 0.07 nM), did not contract either tissue, whereas o-methoxyphenylpiperazide derivative 4F (MPPA-4F), a 5-HT(1B) subtype-selective antagonist (human K(i) = 4.6 +/- 0.6 nM) potently inhibited sumatriptan-induced contraction in the saphenous vein and basilar artery. These results suggested that sumatriptan-induced contraction was mediated via the 5-HT(1B) receptor in these blood vessels. 5-HT(1B) receptor-mediated contraction was then compared in endothelium-intact and denuded vessels to evaluate the role of the endothelium in regulating sumatriptan-induced contractility in these tissues. The presence of an intact endothelium inhibited 5-HT(1B)-induced contraction in both tissues. Endothelial denudation or nitric-oxide synthase inhibition with N(omega) nitro-L-arginine methyl ester (L-NAME) (100 microM) increased the efficacy and potency of sumatriptan in the saphenous vein and basilar artery. Surprisingly, in endothelial-denuded vascular tissues, L-NAME (100 microM) also significantly increased the maximal 5-HT(1B) receptor-induced contraction in both tissues, with no effect on potency of sumatriptan. The effect of L-NAME after endothelial denudation may reflect the presence of a low density of residual endothelial cells as estimated by CD31 antibody staining combined with the modulating effect of nitric oxide released from nonendothelial cells in vascular tissue. Endothelial modulation was specific to 5-HT(1B) receptors because removal of the endothelium did not significantly alter contraction to norepinephrine, histamine, prostaglandin, or potassium chloride in the saphenous vein or basilar artery.


