OxyresveratrolCAS# 29700-22-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 29700-22-9 | SDF | Download SDF |
| PubChem ID | 5281717 | Appearance | Beige powder |
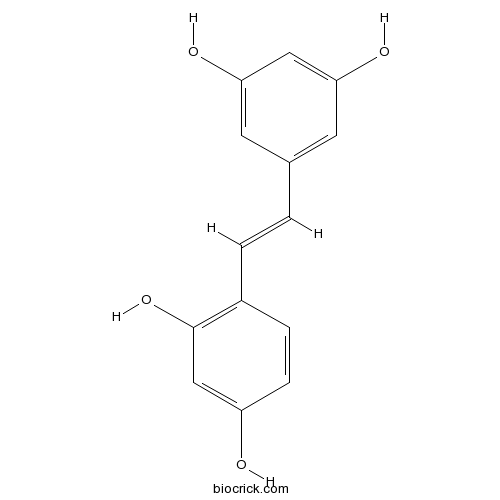
| Formula | C14H12O4 | M.Wt | 244.2 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | trans-Oxyresveratrol | ||
| Solubility | DMSO : ≥ 34 mg/mL (139.21 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-[(E)-2-(3,5-dihydroxyphenyl)ethenyl]benzene-1,3-diol | ||
| SMILES | C1=CC(=C(C=C1O)O)C=CC2=CC(=CC(=C2)O)O | ||
| Standard InChIKey | PDHAOJSHSJQANO-OWOJBTEDSA-N | ||
| Standard InChI | InChI=1S/C14H12O4/c15-11-4-3-10(14(18)8-11)2-1-9-5-12(16)7-13(17)6-9/h1-8,15-18H/b2-1+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Oxyresveratrol , a dietary phenolic compound, has neuroprotective effect, as a potential nutritional candidate for protection against neurodegeneration in Parkinson disease; Oxyresveratrol has antioxidant activity, can reduce neuronal oxidative damage and protect hepatocytes against oxidative stress and mitochondrial dysfunction, which may be associated with activation of Nrf2, it also as an antibrowning agent for cloudy apple juices and fresh-cut apples. Oxyresveratrol exhibits a potent inhibitory effect on dopa oxidase activity of tyrosinase which catalyzes rate-limiting steps of melanin biosynthesis.Oxyresveratrol exhibits the inhibitory activity at the early and late phase of viral replication and inhibited the viral replication with pretreatment in one-step growth assay of HSV-1 and HSV-2. |
| Targets | Caspase | Nrf2 | HO-1 | JNK | ERK | HIV | Tyrosinase | HSV |
| In vitro | Dietary oxyresveratrol prevents parkinsonian mimetic 6-hydroxydopamine neurotoxicity.[Pubmed: 18675900 ]Free Radic Biol Med. 2008 Oct 1;45(7):1019-26.Oxyresveratrol (OXY) is a polyhydroxylated stilbene existing in mulberry. Increasing lines of evidence have shown its neuroprotective effects against Alzheimer disease and stroke. However, little is known about its neuroprotective effect in Parkinson disease (PD).
Owing to its antioxidant activity, blood-brain barrier permeativity, and water solubility, we hypothesized that OXY may exert neuroprotective effects against parkinsonian mimetic 6-hydroxydopamine (6-OHDA) neurotoxicity.
Potential neuroprotective effects of oxyresveratrol against traumatic injury.[Pubmed: 22489319]Eur J Pharmacol. 2012 Apr 5;680(1-3):55-62.Oxyresveratrol is a potent antioxidant and free-radical scavenger found in mulberry wood (Morus alba L.) with demonstrated protective effects against cerebral ischemia.
Oxyresveratrol as an antibrowning agent for cloudy apple juices and fresh-cut apples.[Pubmed: 17335224]J Agric Food Chem. 2007 Apr 4;55(7):2604-10.Antibrowning activities of Morus alba L. twig extracts, Oxyresveratrol, and mulberroside A isolated from mulberry twig on cloudy apple juices and fresh-cut apple slices were evaluated by monitoring the change of a* value, total color difference (DeltaE), and visual observation.
|
| In vivo | Oxyresveratrol abrogates oxidative stress by activating ERK-Nrf2 pathway in the liver.[Pubmed: 26102008]Chem Biol Interact. 2016 Feb 5;245:110-21.Oxyresveratrol is a polyphenolic phytoalexin produced by plants as an antioxidant. This study investigated the hepatoprotective effects of Oxyresveratrol as well as its underlying mechanism of action.
|
| Cell Research | The protective effects of oxyresveratrol imine derivative against hydrogen peroxide-induced cell death in PC12 cells.[Pubmed: 23298159]Free Radic Res. 2013 Mar;47(3):212-8.Oxyresveratrol (2',3,4',5-tetrahydroxystilbene) is a naturally occurring ingredient found in mulberries that shows potential as an antioxidant, anti-inflammatory, and neuroprotective agent.
This study was performed to identify materials similar to Oxyresveratrol that may have more effective antioxidant properties.
|
| Animal Research | Anti-herpes simplex virus (HSV-1) activity of oxyresveratrol derived from Thai medicinal plant: mechanism of action and therapeutic efficacy on cutaneous HSV-1 infection in mice.[Pubmed: 18565600 ]Antiviral Res. 2008 Oct;80(1):62-70.Oxyresveratrol, a major compound purified from Artocarpus lakoocha, a Thai traditional medicinal plant, was evaluated for its mechanism of action and therapeutic efficacy on cutaneous herpes simplex virus (HSV) infection in mice.
|
| Structure Identification | Arch Dermatol Res. 2014 Jul;306(5):475-87.Effects of resveratrol, oxyresveratrol, and their acetylated derivatives on cellular melanogenesis.[Pubmed: 24414332]Resveratrol and Oxyresveratrol are naturally occurring phenolic compounds with various bioactivities, but their uses in cosmetics have been partly limited by their chemical instabilities.
This study was performed to examine the anti-melanogenic effects of the acetylated derivatives from resveratrol and Oxyresveratrol.
|

Oxyresveratrol Dilution Calculator

Oxyresveratrol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.095 mL | 20.475 mL | 40.95 mL | 81.9001 mL | 102.3751 mL |
| 5 mM | 0.819 mL | 4.095 mL | 8.19 mL | 16.38 mL | 20.475 mL |
| 10 mM | 0.4095 mL | 2.0475 mL | 4.095 mL | 8.19 mL | 10.2375 mL |
| 50 mM | 0.0819 mL | 0.4095 mL | 0.819 mL | 1.638 mL | 2.0475 mL |
| 100 mM | 0.041 mL | 0.2048 mL | 0.4095 mL | 0.819 mL | 1.0238 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Oxyresveratrol is neuroprotective and inhibits the apoptotic cell death in transient cerebral ischemia. It effectively scavenges H2O2, NO (IC50 = 45.3 μM), and the artificial free radical 2,2-diphenyl-l-picrylhydrazyl (IC50 = 28.9 μM) In vitro: 1)oxyresveratrol exhibited more than 50% inhibition at 100 μM on L-tyrosine oxidation by murine tyrosinase activity. 2) oxyresveratrol showed an IC50 value of 52.7 μM on the enzyme activity. 3) oxyresveratrol works through reversible inhibition of tyrosinase activity rather than suppression of the expression and synthesis of the enzyme.[2] In vivo: 1) Oxyresveratrol (10 or 20 mg/kg) significantly reduced the brain infarct volume by approximately 54% and 63%, respectively, when compared to vehicle-treated MCAO rats. 2) oxyresveratrol treatment diminished cytochrome c release and decreasedcaspase-3 activation in MCAO rats. [3]
References:
[1]. Lorenz. et al. Oxyresveratrol and resveratrol are potent antioxidants and free radical scavengers: Effect on nitrosative and oxidative stress derived from microglial cells. Nitric Oxide 9(2) 64-76 (2003).
[2]. Kim, Y.M., Yun, J., Lee, C., et al. Oxyresveratrol and hydroxystilbene compounds. Inhbitory effect on tyrosinase and mechanism of action. J Biol Chem277(18) 16340-16344 (2002).
[3]. Shaida A Andrabi et al. Oxyresveratrol (trans-2,3′,4,5′-tetrahydroxystilbene) is neuroprotective and inhibits the apoptotic cell death in transient cerebral ischemia. Brain Res, 2004 Aug 13, 1017(1-2):98-107.
- Ethynodiol diacetate
Catalog No.:BCC4483
CAS No.:297-76-7
- 2-Aminothiazol-4-acetic acid
Catalog No.:BCC8556
CAS No.:29676-71-9
- 13-Oxo-9E,11E-octadecadienoic acid
Catalog No.:BCN8173
CAS No.:29623-29-8
- Friedelin 3,4-lactone
Catalog No.:BCN6449
CAS No.:29621-75-8
- Gynuramide II
Catalog No.:BCN5200
CAS No.:295803-03-1
- 2-Amino-2',5-dichlorobenzophenone
Catalog No.:BCC8520
CAS No.:2958-36-3
- Sakuranetin
Catalog No.:BCN5199
CAS No.:2957-21-3
- (E)-N-Caffeoylputrescine
Catalog No.:BCC8391
CAS No.:29554-26-5
- Negletein
Catalog No.:BCN8085
CAS No.:29550-13-8
- Olivil
Catalog No.:BCN5198
CAS No.:2955-23-9
- Eupatoletin
Catalog No.:BCN3605
CAS No.:29536-44-5
- MNI-caged-L-glutamate
Catalog No.:BCC7086
CAS No.:295325-62-1
- dihydrokaempferol
Catalog No.:BCC8191
CAS No.:5150-32-3
- DOB hydrochloride
Catalog No.:BCC5947
CAS No.:29705-96-2
- Methyl chlorogenate
Catalog No.:BCC9042
CAS No.:29708-87-0
- Clopidol
Catalog No.:BCC8918
CAS No.:2971-90-6
- Dihydro-β-erythroidine hydrobromide
Catalog No.:BCC7341
CAS No.:29734-68-7
- Apigenin-7-glucuronide
Catalog No.:BCN5326
CAS No.:29741-09-1
- Luteolin-7-O-glucuronide
Catalog No.:BCN5338
CAS No.:29741-10-4
- Loganetin
Catalog No.:BCN5202
CAS No.:29748-10-5
- Altretamine hydrochloride
Catalog No.:BCC4114
CAS No.:2975-00-0
- Altenuene
Catalog No.:BCN7392
CAS No.:29752-43-0
- Teniposide
Catalog No.:BCC3864
CAS No.:29767-20-2
- Nudifloside B
Catalog No.:BCN7474
CAS No.:297740-98-8
Anti-herpes simplex virus (HSV-1) activity of oxyresveratrol derived from Thai medicinal plant: mechanism of action and therapeutic efficacy on cutaneous HSV-1 infection in mice.[Pubmed:18565600]
Antiviral Res. 2008 Oct;80(1):62-70.
Oxyresveratrol, a major compound purified from Artocarpus lakoocha, a Thai traditional medicinal plant, was evaluated for its mechanism of action and therapeutic efficacy on cutaneous herpes simplex virus (HSV) infection in mice. The inhibitory concentrations for 50% HSV-1 plaque formation of Oxyresveratrol, three clinical isolates, thymidine kinase (TK)-deficient and phosphonoacetic acid (PAA)-resistant HSV-1 were 19.8, 23.3, 23.5, 24.8, 25.5 and 21.7microg/ml, respectively. Oxyresveratrol exhibited the inhibitory activity at the early and late phase of viral replication and inhibited the viral replication with pretreatment in one-step growth assay of HSV-1 and HSV-2. Oxyresveratrol inhibited late protein synthesis at 30microg/ml. The combination of Oxyresveratrol and acyclovir (ACV) produced synergistic anti-HSV-1 effect, as characterized by the isobologram of plaque inhibition. Mice orally treated with Oxyresveratrol (500mg/kg/dose) dose at 8 h before and three times daily had significant delay in herpetic skin lesion development (P<0.05). Topical application of 30% Oxyresveratrol ointment five times daily significantly delayed the development of skin lesions and protected mice from death (P<0.0001).
The protective effects of oxyresveratrol imine derivative against hydrogen peroxide-induced cell death in PC12 cells.[Pubmed:23298159]
Free Radic Res. 2013 Mar;47(3):212-8.
Oxyresveratrol (2',3,4',5-tetrahydroxystilbene) is a naturally occurring ingredient found in mulberries that shows potential as an antioxidant, anti-inflammatory, and neuroprotective agent. This study was performed to identify materials similar to Oxyresveratrol that may have more effective antioxidant properties. We synthesized a stilbene analog referred to as Compound 1 (2',3,4',5-tetramethoxystilbene); a benzamide analog referred to as Compound 2 ((2,4-dimethoxyphenyl)-3,5-dimethoxybenzamide); and three imine analogs referred to as Compound 3 (3,5-dimethoxybenzylidene)-(2,4-dimethoxyphenylamine), Compound 4 ((4-methoxybenzylidene)-(3-methoxyphenyl)amine), and Compound 5 ((4-methoxybenzylidene)phenylamine). The cytoprotective effects of these compounds were subsequently evaluated using hydrogen peroxide-treated PC12 cells. The cytoprotective effects of the imine analogs were greater than the effects of Oxyresveratrol and the other analogs at concentrations of 200 muM. The Compound 3, which is the most effective imine analog of Oxyresveratrol, exhibited these cytoprotective effects against hydrogen peroxide-induced oxidative stress through the regulation of heme oxygenase-1 (HO-1) expression and the translocation of nuclear factor E2-related factor 2 (Nrf2). Our results suggest that imine analogs of Oxyresveratrol may be useful agents in reducing neuronal oxidative damage.
Potential neuroprotective effects of oxyresveratrol against traumatic injury.[Pubmed:22489319]
Eur J Pharmacol. 2012 Apr 5;680(1-3):55-62.
Oxyresveratrol is a potent antioxidant and free-radical scavenger found in mulberry wood (Morus alba L.) with demonstrated protective effects against cerebral ischemia. We analyzed the neuroprotective ability of Oxyresveratrol using an in vitro model of stretch-induced trauma in co-cultures of neurons and glia, or by exposing cultures to high levels of glutamate. Cultures were treated with 25 muM, 50 muM or 100 muM Oxyresveratrol at the time of injury. Trauma produced marked neuronal death when measured 24 h post-injury, and Oxyresveratrol significantly inhibited this death. Microscopic examination of glia suggested signs of toxicity in cultures treated with 100 muM Oxyresveratrol, as demonstrated by elevated S-100B protein release and a high proportion of cells with condensed nuclei. Cultures exposed to glutamate (100 muM) for 24 h exhibited ~ 37% neuronal loss, which was not inhibited by Oxyresveratrol. These results show that the two pathologies of high glutamate exposure and trauma are differentially affected by Oxyresveratrol treatment in vitro. Further studies using Oxyresveratrol in trauma models are warranted, as toxicity to glia could be beneficial by inhibiting reactive gliosis, which often occurs after trauma.
Dietary oxyresveratrol prevents parkinsonian mimetic 6-hydroxydopamine neurotoxicity.[Pubmed:18675900]
Free Radic Biol Med. 2008 Oct 1;45(7):1019-26.
Oxyresveratrol (OXY) is a polyhydroxylated stilbene existing in mulberry. Increasing lines of evidence have shown its neuroprotective effects against Alzheimer disease and stroke. However, little is known about its neuroprotective effect in Parkinson disease (PD). Owing to its antioxidant activity, blood-brain barrier permeativity, and water solubility, we hypothesized that OXY may exert neuroprotective effects against parkinsonian mimetic 6-hydroxydopamine (6-OHDA) neurotoxicity. Neuroblastoma SH-SY5Y cells have long been used as dopaminergic neurons in PD research. We found that both pretreatment and posttreatment with OXY on SH-SY5Y cells significantly reduced the release of lactate dehydrogenase, the activity of caspase-3, and the generation of intracellular reactive oxygen species triggered by 6-OHDA. Compared to resveratrol, OXY exhibited a wider effective dosage range. We proved that OXY could penetrate the cell membrane by HPLC analysis of cell extracts. These results suggest that OXY may act as an intracellular antioxidant to reduce oxidative stress induced by 6-OHDA. Western blot analysis demonstrated that OXY markedly attenuated 6-OHDA-induced phosphorylation of JNK and c-Jun. Furthermore, we proved that OXY increased the basal levels of SIRT1, which may disclose new pathways accounting for the neuroprotective effects of OXY. Taken together, our results suggest OXY, a dietary phenolic compound, as a potential nutritional candidate for protection against neurodegeneration in PD.
Effects of resveratrol, oxyresveratrol, and their acetylated derivatives on cellular melanogenesis.[Pubmed:24414332]
Arch Dermatol Res. 2014 Jul;306(5):475-87.
Resveratrol and Oxyresveratrol are naturally occurring phenolic compounds with various bioactivities, but their uses in cosmetics have been partly limited by their chemical instabilities. This study was performed to examine the anti-melanogenic effects of the acetylated derivatives from resveratrol and Oxyresveratrol. Resveratrol and Oxyresveratrol were chemically modified to triacetyl resveratrol and tetraacetyl Oxyresveratrol, respectively. The acetylated compounds were less susceptible than the parent compounds to oxidative discoloration. The acetylated compounds inhibited the activities of tyrosinases less than parent compounds in vitro, but they were as effective at cellular melanogenesis inhibition, indicating bioconversion to parent compounds inside cells. Supporting this notion, the parent compounds were regenerated when the acetylated compounds were digested with cell lysates. Although resveratrol and triacetyl resveratrol inhibited tyrosinase activity less effectively than Oxyresveratrol and tetraacetyl Oxyresveratrol in vitro, they inhibited cellular melanogenesis more effectively. This discrepancy was explained by strong inhibition of tyrosinase expression by resveratrol and triacetyl resveratrol. Experiments using a reconstituted skin model indicated that resveratrol derivatives can affect melanin synthesis and cell viability to different extents. Collectively, this study suggests that acetylated derivatives of resveratrol have great potential as anti-melanogenic agents for cosmetic use in terms of efficacy, safety, and stability.
Oxyresveratrol abrogates oxidative stress by activating ERK-Nrf2 pathway in the liver.[Pubmed:26102008]
Chem Biol Interact. 2016 Feb 5;245:110-21.
Oxyresveratrol is a polyphenolic phytoalexin produced by plants as an antioxidant. This study investigated the hepatoprotective effects of Oxyresveratrol as well as its underlying mechanism of action. Here, we evaluated the protective effects of Oxyresveratrol against tert-butyl hydroperoxide (tBHP)-induced severe oxidative stress in HepG2 cells as well as acute liver injury caused by carbon tetrachloride (CCl4) in mice. tBHP-induced reactive oxygen species production and cell death in hepatocytes were blocked by Oxyresveratrol, as indicated by MTT, TUNEL, and FACS analyses. Moreover, pretreatment with Oxyresveratrol increased nuclear translocation and transactivation of NF-E2-related factor 2 (Nrf2), as assessed by antioxidant response element reporter gene expression and immunofluorescence staining, and transactivated expression of both hemeoxygenase-1 and glutamate-cysteine ligase catalytic subunit. More importantly, Oxyresveratrol induced phosphorylation of Nrf2 mediated through activation of extracellular signal-regulated kinase 1/2 (ERK1/2). Further, ERK inhibitors such as PD98059 and U0126 blocked phosphorylation of Nrf2 as well as the protective effect of Oxyresveratrol in mitochondria. In mice, oral administration of Oxyresveratrol significantly prevented hepatocyte degeneration, inflammatory cell infiltration, as well as elevation of plasma markers such as ALT and AST induced by CCl4 injection. In conclusion, this study confirmed that Oxyresveratrol protected hepatocytes against oxidative stress and mitochondrial dysfunction, which might be associated with activation of Nrf2.
Oxyresveratrol as an antibrowning agent for cloudy apple juices and fresh-cut apples.[Pubmed:17335224]
J Agric Food Chem. 2007 Apr 4;55(7):2604-10.
Antibrowning activities of Morus alba L. twig extracts, Oxyresveratrol, and mulberroside A isolated from mulberry twig on cloudy apple juices and fresh-cut apple slices were evaluated by monitoring the change of a* value, total color difference (DeltaE), and visual observation. It was found, similar to 4-hexylresorcinol, that Oxyresveratrol could effectively inhibit browning in cloudy apple juices at a concentration as low as 0.01% and that mulberry twig extract also showed remarkable antibrowning effects on cloudy apple juices. However, for fresh-cut apple slices, mulberry twig extract and Oxyresveratrol needed to be used in combination at least with ascorbic acid to exhibit their antibrowning effects. Apple slice samples treated by dipping in a solution containing 0.001 M Oxyresveratrol, 0.5 M isoascorbic acid, 0.05 M calcium chloride, and 0.025 M acetylcysteine did not undergo any substantial browning reaction for 28 days at 4 degrees C. However mulberroside A did not show antibrowning effects on cloudy apple juices although it is also a good mushroom tyrosinase inhibitor.


