dihydrokaempferolCAS# 5150-32-3 |
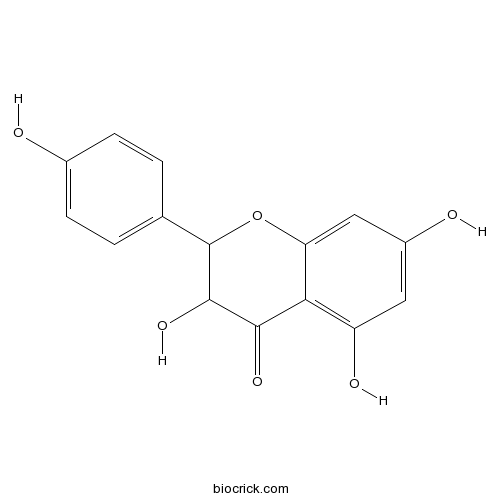
- Aromadendrin
Catalog No.:BCN5552
CAS No.:480-20-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 5150-32-3 | SDF | Download SDF |
| PubChem ID | 662 | Appearance | Powder |
| Formula | C15H12O6 | M.Wt | 288.26 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Aromadedrin;3,4',5,7-tetrahydroxyflavanone | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one | ||
| SMILES | C1=CC(=CC=C1C2C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O | ||
| Standard InChIKey | PADQINQHPQKXNL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H12O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,14-18,20H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

dihydrokaempferol Dilution Calculator

dihydrokaempferol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4691 mL | 17.3455 mL | 34.6909 mL | 69.3818 mL | 86.7273 mL |
| 5 mM | 0.6938 mL | 3.4691 mL | 6.9382 mL | 13.8764 mL | 17.3455 mL |
| 10 mM | 0.3469 mL | 1.7345 mL | 3.4691 mL | 6.9382 mL | 8.6727 mL |
| 50 mM | 0.0694 mL | 0.3469 mL | 0.6938 mL | 1.3876 mL | 1.7345 mL |
| 100 mM | 0.0347 mL | 0.1735 mL | 0.3469 mL | 0.6938 mL | 0.8673 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Oxyresveratrol
Catalog No.:BCN5201
CAS No.:29700-22-9
- Ethynodiol diacetate
Catalog No.:BCC4483
CAS No.:297-76-7
- 2-Aminothiazol-4-acetic acid
Catalog No.:BCC8556
CAS No.:29676-71-9
- 13-Oxo-9E,11E-octadecadienoic acid
Catalog No.:BCN8173
CAS No.:29623-29-8
- Friedelin 3,4-lactone
Catalog No.:BCN6449
CAS No.:29621-75-8
- Gynuramide II
Catalog No.:BCN5200
CAS No.:295803-03-1
- 2-Amino-2',5-dichlorobenzophenone
Catalog No.:BCC8520
CAS No.:2958-36-3
- Sakuranetin
Catalog No.:BCN5199
CAS No.:2957-21-3
- (E)-N-Caffeoylputrescine
Catalog No.:BCC8391
CAS No.:29554-26-5
- Negletein
Catalog No.:BCN8085
CAS No.:29550-13-8
- Olivil
Catalog No.:BCN5198
CAS No.:2955-23-9
- Eupatoletin
Catalog No.:BCN3605
CAS No.:29536-44-5
- DOB hydrochloride
Catalog No.:BCC5947
CAS No.:29705-96-2
- Methyl chlorogenate
Catalog No.:BCC9042
CAS No.:29708-87-0
- Clopidol
Catalog No.:BCC8918
CAS No.:2971-90-6
- Dihydro-β-erythroidine hydrobromide
Catalog No.:BCC7341
CAS No.:29734-68-7
- Apigenin-7-glucuronide
Catalog No.:BCN5326
CAS No.:29741-09-1
- Luteolin-7-O-glucuronide
Catalog No.:BCN5338
CAS No.:29741-10-4
- Loganetin
Catalog No.:BCN5202
CAS No.:29748-10-5
- Altretamine hydrochloride
Catalog No.:BCC4114
CAS No.:2975-00-0
- Altenuene
Catalog No.:BCN7392
CAS No.:29752-43-0
- Teniposide
Catalog No.:BCC3864
CAS No.:29767-20-2
- Nudifloside B
Catalog No.:BCN7474
CAS No.:297740-98-8
- Nudifloside C
Catalog No.:BCN7491
CAS No.:297740-99-9
Effectiveness of Prenyl Group on Flavonoids from Epimedium koreanum Nakai on Bacterial Neuraminidase Inhibition.[Pubmed:30654565]
Molecules. 2019 Jan 16;24(2). pii: molecules24020317.
In this study, the inhibitory potential of bacterial neuraminidase (NA) was observed on the leaves of Epimedium koreanum Nakai, which is a popular ingredient in traditional herbal medicine. This study attempted to isolate the relevant, responsible metabolites and elucidate their inhibition mechanism. The methanol extraction process yielded eight flavonoids (1(-)8), of which compounds 7 and 8 were new compounds named koreanoside F and koreanoside G, respectively. All the compounds (1(-)8) showed a significant inhibition to bacterial NA with IC50 values of 0.17(-)106.3 microM. In particular, the prenyl group on the flavonoids played a critical role in bacterial NA inhibition. Epimedokoreanin B (compound 1, IC50 = 0.17 microM) with two prenyl groups on C8 and C5' of luteolin was 500 times more effective than luteolin (IC50 = 85.6 microM). A similar trend was observed on compound 2 (IC50 = 0.68 microM) versus dihydrokaempferol (IC50 = 500.4 microM) and compound 3 (IC50 = 12.6 microM) versus apigenin (IC50 = 107.5 microM). Kinetic parameters (Km, Vmax, and Kik/Kiv) evaluated that all the compounds apart from compound 5 showed noncompetitive inhibition. Compound 5 was proven to be a mixed type inhibitor. In an enzyme binding affinity experiment using fluorescence, affinity constants (KSV) were tightly related to inhibitory activities.
Apoptosis Effects of Dihydrokaempferol Isolated from Bauhinia championii on Synoviocytes.[Pubmed:30622621]
Evid Based Complement Alternat Med. 2018 Dec 2;2018:9806160.
Bauhinia championii (Benth.) Benth. is a traditional medicinal plant used in China to treat rheumatoid arthritis (RA), especially in She ethnic minority group. This study focused on the active constituents from the rattan of B. championii (Benth.) Benth., which possess potential apoptosis effects. A conventional phytochemical separation method for the isolation of compounds from the ethyl acetate extract of B. championii was developed. The procedure involved extraction, liquid-liquid partitioning with ethyl acetate, and subsequent compound purification, respectively. Additionally, cell viability of dihydrokaempferol found abundantly in it was evaluated in vitro by MTS, and the antiapoptosis effect was evaluated by annexin V/PI staining (Flow Cytometry Analysis) and western blot. The results showed that nine flavonoids, and five other compounds, were isolated from the ethyl acetate extract of B. championii and were identified as beta-sitosterol (1), 5,6,7,3',4',5'-hexamethoxyflavone (2), 3',4',5,7-tetrahydroxyflavone (3), 5,7,3',4',5'-pentamethoxyflavone (4), 4'-hydroxy-5,7,3',5'-pentamethoxyflavone (5), apigenin (6), liquiritigenin (7), 5, 7-dihydroxylcoumarin (8), 3',4',5,7, -pentamethoxyflavone (9), n-octadecanoate (10), lupine ketone (11), dibutylphthalate (12), dihydrokaempferol (13), and 5,7,3',5'-tetrahydroxy-6-methylflavanone (14). Among these compounds, 5-14 were isolated for the first time from B. championii. In addition, apoptosis effects of abundant dihydrokaempferol were evaluated in vitro. dihydrokaempferol exhibited inhibitory effects on the proliferation of synoviocytes. Furthermore, dihydrokaempferol promoted Bax and Bad expression, as well as the cleavage of caspase-9, caspase-3, and PARP. Meanwhile, it inhibited Bcl-2 and Bcl-xL expression. These findings indicate that dihydrokaempferol isolated from the ethyl acetate extract of B. championii effectively promotes apoptosis, which is an important process through suppression of apoptotic activity. The results are encouraging for further studies on the use of B. championii in the treatment of RA.
The Peroxidative Cleavage of Kaempferol Contributes to the Biosynthesis of the Benzenoid Moiety of Ubiquinone in Plants.[Pubmed:30429224]
Plant Cell. 2018 Dec;30(12):2910-2921.
Land plants possess the unique capacity to derive the benzenoid moiety of the vital respiratory cofactor, ubiquinone (coenzyme Q), from phenylpropanoid metabolism via beta-oxidation of p-coumarate to form 4-hydroxybenzoate. Approximately half of the ubiquinone in plants comes from this pathway; the origin of the rest remains enigmatic. In this study, Phe-[Ring-(13)C6] feeding assays and gene network reconstructions uncovered a connection between the biosynthesis of ubiquinone and that of flavonoids in Arabidopsis (Arabidopsis thaliana). Quantification of ubiquinone in Arabidopsis and tomato (Solanum lycopersicum) mutants in flavonoid biosynthesis pinpointed the corresponding metabolic branch-point as lying between flavanone-3-hydroxylase and flavonoid-3'-hydroxylase. Further isotopic labeling and chemical rescue experiments demonstrated that the B-ring of kaempferol is incorporated into ubiquinone. Moreover, heme-dependent peroxidase activities were shown to be responsible for the cleavage of B-ring of kaempferol to form 4-hydroxybenzoate. By contrast, kaempferol 3-beta-d-glucopyranoside, dihydrokaempferol, and naringenin were refractory to peroxidative cleavage. Collectively, these data indicate that kaempferol contributes to the biosynthesis of a vital respiratory cofactor, resulting in an extraordinary metabolic arrangement where a specialized metabolite serves as a precursor for a primary metabolite. Evidence is also provided that the ubiquinone content of tomato fruits can be manipulated via deregulation of flavonoid biosynthesis.
The rare orange-red colored Euphorbia pulcherrima cultivar 'Harvest Orange' shows a nonsense mutation in a flavonoid 3'-hydroxylase allele expressed in the bracts.[Pubmed:30285622]
BMC Plant Biol. 2018 Oct 3;18(1):216.
BACKGROUND: Commercially available poinsettia (Euphorbia pulcherrima) varieties prevalently accumulate cyanidin derivatives and show intense red coloration. Orange-red bract color is less common. We investigated four cultivars displaying four different red hues with respect to selected enzymes and genes of the anthocyanin pathway, putatively determining the color hue. RESULTS: Red hues correlated with anthocyanin composition and concentration and showed common dark red coloration in cultivars 'Christmas Beauty' and 'Christmas Feeling' where cyanidin derivatives were prevalent. In contrast, orange-red bract color is based on the prevalent presence of pelargonidin derivatives that comprised 85% of the total anthocyanin content in cv. 'Premium Red' and 96% in cv. 'Harvest Orange' (synonym: 'Orange Spice'). cDNA clones of flavonoid 3'-hydroxylase (F3'H) and dihydroflavonol 4-reductase (DFR) were isolated from the four varieties, and functional activity and substrate specificity of the corresponding recombinant enzymes were studied. Kinetic studies demonstrated that poinsettia DFRs prefer dihydromyricetin and dihydroquercetin over dihydrokaempferol, and thus, favor the formation of cyanidin over pelargonidin. Whereas the F3'H cDNA clones of cultivars 'Christmas Beauty', 'Christmas Feeling', and 'Premium Red' encoded functionally active enzymes, the F3'H cDNA clone of cv. 'Harvest Orange' contained an insertion of 28 bases, which is partly a duplication of 20 bases found close to the insertion site. This causes a frameshift mutation with a premature stop codon after nucleotide 132 and, therefore, a non-functional enzyme. Heterozygosity of the F3'H was demonstrated in this cultivar, but only the mutated allele was expressed in the bracts. No correlation between F3'H-expression and the color hue could be observed in the four species. CONCLUSIONS: Rare orange-red poinsettia hues caused by pelargonidin based anthocyanins can be achieved by different mechanisms. F3'H is a critical step in the establishment of orange red poinsettia color. Although poinsettia DFR shows a low substrate specificity for dihydrokaempferol, sufficient precursor for pelargonidin formation is available in planta, in the absence of F3'H activity.
Constitutive Polyphenols in Blades and Veins of Grapevine ( Vitis vinifera L.) Healthy Leaves.[Pubmed:30175914]
J Agric Food Chem. 2018 Oct 24;66(42):10977-10990.
Despite the economic importance and the diffusion of grapevine cultivation worldwide, little is known about leaf chemical composition. We characterized the phenolic composition of Nebbiolo, Barbera, Pinot noir, Cabernet Sauvignon, Grenache, and Shiraz ( Vitis vinifera L.) healthy leaves (separating blades and veins) during the season. Quantitative and qualitative differences were found between leaf sectors and among genotypes. In healthy grapevine leaves, anthocyanins, dihydromyricetin-rhamnoside, hexosides of dihydroquercetin, and dihydrokaempferol exclusively accumulated in veins. Astilbin was the only flavanonol detected in blades and the prevalent flavanonol in veins. Barbera distinguished for the lowest proanthocyanidin and the highest hydroxycinnamate content, and Pinot noir for the absence of acylated-anthocyanins. Nebbiolo, and Cabernet Sauvignon displayed a high concentration of epigallocatechin gallate in veins. Nebbiolo leaves showed the highest concentrations of flavanonols and the widest profile differentiation. Knowledge derived from the present work is a contribution to find out leaf polyphenol potential as a part of grapevine defense mechanisms and to dissect genotype-related susceptibility to pathogens; moreover, it represents a starting point for future deepening about grapevine and vineyard byproducts as a source of bioactive phenolic compounds.
Engineering de novo anthocyanin production in Saccharomyces cerevisiae.[Pubmed:29970082]
Microb Cell Fact. 2018 Jul 3;17(1):103.
BACKGROUND: Anthocyanins are polyphenolic pigments which provide pink to blue colours in fruits and flowers. There is an increasing demand for anthocyanins, as food colorants and as health-promoting substances. Plant production of anthocyanins is often seasonal and cannot always meet demand due to low productivity and the complexity of the plant extracts. Therefore, a system of on-demand supply is useful. While a number of other (simpler) plant polyphenols have been successfully produced in the yeast Saccharomyces cerevisiae, production of anthocyanins has not yet been reported. RESULTS: Saccharomyces cerevisiae was engineered to produce pelargonidin 3-O-glucoside starting from glucose. Specific anthocyanin biosynthetic genes from Arabidopsis thaliana and Gerbera hybrida were introduced in a S. cerevisiae strain producing naringenin, the flavonoid precursor of anthocyanins. Upon culturing, pelargonidin and its 3-O-glucoside were detected inside the yeast cells, albeit at low concentrations. A number of related intermediates and side-products were much more abundant and were secreted into the culture medium. To optimize titers of pelargonidin 3-O-glucoside further, biosynthetic genes were stably integrated into the yeast genome, and formation of a major side-product, phloretic acid, was prevented by engineering the yeast chassis. Further engineering, by removing two glucosidases which are known to degrade pelargonidin 3-O-glucoside, did not result in higher yields of glycosylated pelargonidin. In aerated, pH controlled batch reactors, intracellular pelargonidin accumulation reached 0.01 micromol/gCDW, while kaempferol and dihydrokaempferol were effectively exported to reach extracellular concentration of 20 microM [5 mg/L] and 150 microM [44 mg/L], respectively. CONCLUSION: The results reported in this study demonstrate the proof-of-concept that S. cerevisiae is capable of de novo production of the anthocyanin pelargonidin 3-O-glucoside. Furthermore, while current conversion efficiencies are low, a number of clear bottlenecks have already been identified which, when overcome, have huge potential to enhance anthocyanin production efficiency. These results bode very well for the development of fermentation-based production systems for specific and individual anthocyanin molecules. Such systems have both great scientific value for identifying and characterising anthocyanin decorating enzymes as well as significant commercial potential for the production of, on-demand, pure bioactive compounds to be used in the food, health and even pharma industries.
Melatonin Antagonizes Jasmonate-Triggered Anthocyanin Biosynthesis in Arabidopsis thaliana.[Pubmed:29758982]
J Agric Food Chem. 2018 May 30;66(21):5392-5400.
As a plant-specific flavonoid type metabolite, anthocyanin is an important plant-sourced nutrition. Although the anthocyanin biosynthesis pathway has been revealed, how to modulate anthocyanin production by endogenous molecules is still elusive. Here, we investigated the role of melatonin in anthocyanin biosynthesis in the reference plant Arabidopsis thaliana and found that melatonin suppresses anthocyanin synthesis. Moreover, melatonin was able to significantly inhibit jasmonate-stimulated anthocyanin production. Unexpectedly, melatonin could not repress the jasmonate-triggered JAZ protein degradation that is a key event for relaying jasmonate signaling. The expression of jasmonate-induced marker genes or other jasmonate-related phenotypes were not discernibly changed in the presence of melatonin. These results indicate that the antagonization of jasmonate-induced anthocyanin synthesis by melatonin does not occur through the abrogation of jasmonate signaling. Furthermore, we found that melatonin does not trigger anthocyanin catabolism. Finally, we supplied anthocyanin biosynthesis precursors to examine their roles in anthocyanin biosynthesis and found that melatonin most likely acts before the dihydrokaempferol production step. Our work illustrates that melatonin plays a negative role in the induction of anthocyanin biosynthesis and sheds new light on the role of melatonin in plant cell metabolism.
Protective and Therapeutic Effects of Engeletin on LPS-Induced Acute Lung Injury.[Pubmed:29704150]
Inflammation. 2018 Aug;41(4):1259-1265.
Acute lung injury (ALI) is a serious disease with morbidity and mortality in patients. Engeletin (dihydrokaempferol 3-rhamnoside) is a flavanonol glycoside. It can be found in the skin of white grapes and white wine and is widely distributed in southeast Asia. In our study, we evaluated the protective and therapeutic effects of engeletin on lipopolysaccharide (LPS)-induced ALI in animal model. We determined the level of peroxisome proliferator-activated receptor-gamma (PPAR-gamma), nuclear factor kappaB (NF-kappaB), and IkappaBalpha by western blotting. The myeloperoxidase (MPO) activity and lung wet/dry (W/D) ratio in lung tissues were also detected. Histopathological changes and the pro-inflammatory cytokines TNF-alpha, IL-6, and IL-1beta were determined by H&E staining and ELISA. The MPO activity and lung W/D ratio induced by LPS were attenuated by engeletin. The numbers of inflammatory cells and the levels of inflammatory cytokines in bronchoalveolar lavage fluid (BALF) were ameliorated by engeletin. Furthermore, the results also showed that engeletin significantly suppressed LPS-induced NF-kappaB activation. The expression of PPAR-gamma was upregulated by treatment of engeletin. In conclusion, we found that engeletin had protective and therapeutic effects against LPS-induced ALI by activating PPAR-gamma. Engeletin is a potentially effective agent for the treatment of lung injury.
Molecular Cloning and Functional Characterization of a Dihydroflavonol 4-Reductase from Vitis bellula.[Pubmed:29642567]
Molecules. 2018 Apr 10;23(4). pii: molecules23040861.
Vitis bellula is a new grape crop in southern China. Berries of this species are rich in antioxidative anthocyanins and proanthocyanidins. This study reports cloning and functional characterization of a cDNA encoding a V. bellula dihydroflavonol reductase (VbDFR) involved in the biosynthesis of anthocyanins and proanthocyanidins. A cDNA including 1014 bp was cloned from young leaves and its open reading frame (ORF) was deduced encoding 337 amino acids, highly similar to V. vinifera DFR (VvDFR). Green florescence protein fusion and confocal microscopy analysis determined the cytosolic localization of VbDFR in plant cells. A soluble recombinant VbDFR was induced and purified from E. coli for enzyme assay. In the presence of NADPH, the recombinant enzyme catalyzed dihydrokaempferol (DHK) and dihydroquercetin (DHQ) to their corresponding leucoanthocyanidins. The VbDFR cDNA was introduced into tobacco plants via Agrobacterium-mediated transformation. The overexpression of VbDFR increased anthocyanin production in flowers. Anthocyanin hydrolysis and chromatographic analysis revealed that transgenic flowers produced pelargonidin and delphinidin, which were not detected in control flowers. These data demonstrated that the overexpression of VbDFR produced new tobacco anthocyanidins. In summary, all data demonstrate that VbDFR is a useful gene to provide three types of substrates for metabolic engineering of anthocyanins and proanthocyanidins in grape crops and other crops.
Validated HPTLC Method for Dihydrokaempferol-4'-O-glucopyranoside Quantitative Determination in Alcea Species.[Pubmed:29635436]
J Chromatogr Sci. 2018 Jul 1;56(6):518-523.
dihydrokaempferol-4'-O-glucopyranoside, a flavanonol glucoside, is the major compound in the flower of Alcea rosea L. which possesses significant antioxidant and anticancer activity against HepG-2 cell line and thus can be considered a marker compound for A. rosea L. We attempted to establish a new simple, validated high-performance thin-layer chromatographic (HPTLC) method for the quantitation of dihydrokaempferol-4'-O-glucopyranoside to help in the standardization of the hydroalcoholic extracts of A. rosea L. flowers and to evaluate the best method for its extraction from the plant material. The separation was carried out on an HPTLC aluminum plate pre-coated with silica gel 60F-254, eluted with ethyl acetate-methanol-water-acetic acid (30:5:4:0.15 v/v). Densitometric scanning was performed using a Camag TLC scanner III, at 295 nm. A linear relationship was obtained between the concentrations (0.9-3.6 mg) and peak areas with the correlation coefficient (r) of 0.9971 +/- 0.0002. The percentage relative standard deviations of intra-day and inter-day precisions were 0.22-1.45 and 0.49-1.66, respectively. The percentage w/w of dihydrokaempferol-4'-O-glucopyranoside in the flowers of A. rosea L. after maceration and sonication for 15 min was found to be 0.733 g/100 g and 0.928 g/100 g, respectively.
Reduction of Dihydrokaempferol by Vitis vinfera Dihydroflavonol 4-Reductase to Produce Orange Pelargonidin-Type Anthocyanins.[Pubmed:29554804]
J Agric Food Chem. 2018 Apr 4;66(13):3524-3532.
Vitis vinifera has been thought to be unable to produce pelargonidin-type anthocyanins because its dihydroflavonol 4-reductase (DFR) does not efficiently reduce dihydrokaempferol. However, in this study, pelargonidin 3- O-glucoside was detected in the skins of V. vinifera 'Pinot Noir', 'Cabernet Sauvignon', and 'Yan73', as well as in the flesh of 'Yan73' by HPLC-ESI-MS/MS. Additionally, pelargonidin 3- O-(6-acetyl)-glucoside was detected in 'Yan73' skin and flesh for the first time. To further confirm the presence of pelargonidin-type anthocyanins in these grape cultivars, their DFRs were cloned, expressed in Escherichia coli, and purified. An enzyme-activity analysis revealed that V. vinifera DFR can reduce dihydrokaempferol to produce leucopelargonidin, although it prefers dihydroquercetin and dihydromyricetin as substrates. Thus, the existence of a pelargonidin-based anthocyanin-biosynthetic pathway was confirmed in V. vinifera via mass-spectrometric and enzymatic methods and redirected anthocyanin biosynthesis in V. vinifera L. cultivars.
Great Cause-Small Effect: Undeclared Genetically Engineered Orange Petunias Harbor an Inefficient Dihydroflavonol 4-Reductase.[Pubmed:29541079]
Front Plant Sci. 2018 Feb 28;9:149.
A recall campaign for commercial, orange flowering petunia varieties in spring 2017 caused economic losses worldwide. The orange varieties were identified as undeclared genetically engineered (GE)-plants, harboring a maize dihydroflavonol 4-reductase (DFR, A1), which was used in former scientific transgenic breeding attempts to enable formation of orange pelargonidin derivatives from the precursor dihydrokaempferol (DHK) in petunia. How and when the A1 cDNA entered the commercial breeding process is unclear. We provide an in-depth analysis of three orange petunia varieties, released by breeders from three countries, with respect to their transgenic construct, transcriptomes, anthocyanin composition, and flavonoid metabolism at the level of selected enzymes and genes. The two possible sources of the A1 cDNA in the undeclared GE-petunia can be discriminated by PCR. A special version of the A1 gene, the A1 type 2 allele, is present, which includes, at the 3'-end, an additional 144 bp segment from the non-viral transposable Cin4-1 sequence, which does not add any functional advantage with respect to DFR activity. This unequivocally points at the first scientific GE-petunia from the 1980s as the A1 source, which is further underpinned e.g., by the presence of specific restriction sites, parts of the untranslated sequences, and the same arrangement of the building blocks of the transformation plasmid used. Surprisingly, however, the GE-petunia cannot be distinguished from native red and blue varieties by their ability to convert DHK in common in vitro enzyme assays, as DHK is an inadequate substrate for both the petunia and maize DFR. Recombinant maize DFR underpins the low DHK acceptance, and, thus, the strikingly limited suitability of the A1 protein for a transgenic approach for breeding pelargonidin-based flower color. The effect of single amino acid mutations on the substrate specificity of DFRs is demonstrated. Expression of the A1 gene is generally lower than the petunia DFR expression despite being under the control of the strong, constitutive p35S promoter. We show that a rare constellation in flavonoid metabolism-absence or strongly reduced activity of both flavonol synthase and B-ring hydroxylating enzymes-allows pelargonidin formation in the presence of DFRs with poor DHK acceptance.


