Methyl chlorogenateCAS# 29708-87-0 |
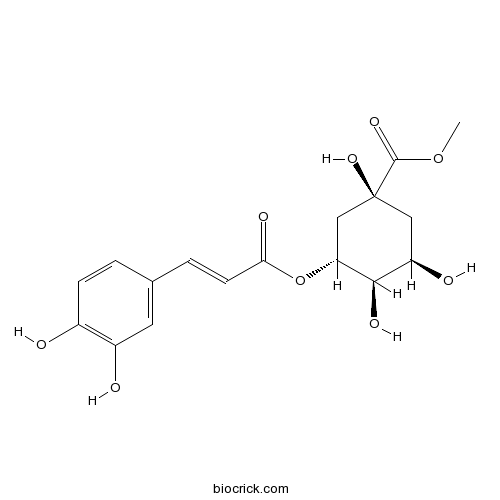
- 3-O-Caffeoylquinic acid methyl ester
Catalog No.:BCN3484
CAS No.:123483-19-2
- Neochlorogenic acid methyl ester
Catalog No.:BCN8860
CAS No.:123410-65-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 29708-87-0 | SDF | Download SDF |
| PubChem ID | 487435 | Appearance | Powder |
| Formula | C17H20O9 | M.Wt | 368.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl (1S,3R,4R,5R)-3-[3-(3,4-dihydroxyphenyl)prop-2-enoyloxy]-1,4,5-trihydroxycyclohexane-1-carboxylate | ||
| SMILES | COC(=O)C1(CC(C(C(C1)OC(=O)C=CC2=CC(=C(C=C2)O)O)O)O)O | ||
| Standard InChIKey | MZNIJRAPCCELQX-SMKXDYDZSA-N | ||
| Standard InChI | InChI=1S/C17H20O9/c1-25-16(23)17(24)7-12(20)15(22)13(8-17)26-14(21)5-3-9-2-4-10(18)11(19)6-9/h2-6,12-13,15,18-20,22,24H,7-8H2,1H3/t12-,13-,15-,17+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Methyl chlorogenate Dilution Calculator

Methyl chlorogenate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7152 mL | 13.5759 mL | 27.1518 mL | 54.3036 mL | 67.8794 mL |
| 5 mM | 0.543 mL | 2.7152 mL | 5.4304 mL | 10.8607 mL | 13.5759 mL |
| 10 mM | 0.2715 mL | 1.3576 mL | 2.7152 mL | 5.4304 mL | 6.7879 mL |
| 50 mM | 0.0543 mL | 0.2715 mL | 0.543 mL | 1.0861 mL | 1.3576 mL |
| 100 mM | 0.0272 mL | 0.1358 mL | 0.2715 mL | 0.543 mL | 0.6788 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- DOB hydrochloride
Catalog No.:BCC5947
CAS No.:29705-96-2
- dihydrokaempferol
Catalog No.:BCC8191
CAS No.:5150-32-3
- Oxyresveratrol
Catalog No.:BCN5201
CAS No.:29700-22-9
- Ethynodiol diacetate
Catalog No.:BCC4483
CAS No.:297-76-7
- 2-Aminothiazol-4-acetic acid
Catalog No.:BCC8556
CAS No.:29676-71-9
- 13-Oxo-9E,11E-octadecadienoic acid
Catalog No.:BCN8173
CAS No.:29623-29-8
- Friedelin 3,4-lactone
Catalog No.:BCN6449
CAS No.:29621-75-8
- Gynuramide II
Catalog No.:BCN5200
CAS No.:295803-03-1
- 2-Amino-2',5-dichlorobenzophenone
Catalog No.:BCC8520
CAS No.:2958-36-3
- Sakuranetin
Catalog No.:BCN5199
CAS No.:2957-21-3
- (E)-N-Caffeoylputrescine
Catalog No.:BCC8391
CAS No.:29554-26-5
- Negletein
Catalog No.:BCN8085
CAS No.:29550-13-8
- Clopidol
Catalog No.:BCC8918
CAS No.:2971-90-6
- Dihydro-β-erythroidine hydrobromide
Catalog No.:BCC7341
CAS No.:29734-68-7
- Apigenin-7-glucuronide
Catalog No.:BCN5326
CAS No.:29741-09-1
- Luteolin-7-O-glucuronide
Catalog No.:BCN5338
CAS No.:29741-10-4
- Loganetin
Catalog No.:BCN5202
CAS No.:29748-10-5
- Altretamine hydrochloride
Catalog No.:BCC4114
CAS No.:2975-00-0
- Altenuene
Catalog No.:BCN7392
CAS No.:29752-43-0
- Teniposide
Catalog No.:BCC3864
CAS No.:29767-20-2
- Nudifloside B
Catalog No.:BCN7474
CAS No.:297740-98-8
- Nudifloside C
Catalog No.:BCN7491
CAS No.:297740-99-9
- Silydianin
Catalog No.:BCN2388
CAS No.:29782-68-1
- Carbamazepine
Catalog No.:BCC4378
CAS No.:298-46-4
In vitro assessment of Argemone mexicana, Taraxacum officinale, Ruta chalepensis and Tagetes filifolia against Haemonchus contortus nematode eggs and infective (L3) larvae.[Pubmed:28578091]
Microb Pathog. 2017 Aug;109:162-168.
Argemone mexicana, Taraxacum officinale, Ruta chalepensis and Tagetes filifolia are plants with deworming potential. The purpose of this study was to evaluate methanolic extracts of aerial parts of these plants against Haemonchus contortus eggs and infective larvae (L3) and identify compounds responsible for the anthelmintic activity. In vitro probes were performed to identify the anthelmintic activity of plant extracts: egg hatching inhibition (EHI) and larvae mortality. Open column Chromatography was used to bio-guided fractionation of the extract, which shows the best anthelmintic effect. The lethal concentration to inhibit 50% of H. contortus egg hatching or larvae mortality (LC50) was calculated using a Probit analysis. Bio-guided procedure led to the recognition of an active fraction (TF11) mainly composed by 1) quercetagitrin, 2) Methyl chlorogenate and chlorogenic acid. Quercetagitrin (1) and Methyl chlorogenate (2) did not show an important EHI activity (3-14%) (p < 0.05); however, chlorogenic acid (3) showed 100% of EHI (LC50 248 mug/mL) (p < 0.05). Chlorogenic acid is responsible of the ovicidal activity and it seems that, this compound is reported for the first time with anthelmintic activity against a parasite of importance in sheep industry.
Inhibitory Activities of Phenolic Compounds Isolated from Adina rubella Leaves Against 5alpha-Reductase Associated with Benign Prostatic Hypertrophy.[Pubmed:27399661]
Molecules. 2016 Jul 7;21(7). pii: molecules21070887.
Adina rubella Hance (AR), a plant native to Korea, has been used as traditional medicine for dysentery, eczema, intoxication, and external hemorrhages. Previous phytochemical studies of AR have reported several components, including terpenoids, phenolics, and alkaloids. The current study evaluated the anti-oxidative and anti-inflammatory activities and 5alpha-reductase inhibition of isolated compounds of AR leaves to find a potential therapeutic agent for benign prostatic hypertrophy (BPH). Repeated chromatographic isolation of an 80% acetone extract of AR leaves yielded seven phenolic compounds: caffeic acid (1), chlorogenic acid (2), Methyl chlorogenate (3), quercetin-3-rutinoside (4), kaempferol-3-O-alpha-l-rhamnopyranosyl-(1-->6)-beta-d-glucopyranoside (5), hyperoside (6), and grandifloroside (7). Compound 7 is a novel compound in AR. Caffeoyl derivatives 1-3 and 7 showed good anti-oxidative activities. In particular, caffeic acid (1) and grandifloroside (7) showed potent anti-inflammatory activities, and 7 also exhibited potent inhibitory activity against TNF-alpha and 5alpha-reductase. Our results show that the extract and grandifloroside (7) from leaves of AR might be developed as a source of potent anti-oxidative and anti-inflammatory agents and therapeutic agent for BPH.
In vitro Cytotoxic Activities and Molecular Mechanisms of Angelica shikokiana Extract and its Isolated Compounds.[Pubmed:27013795]
Pharmacogn Mag. 2015 Oct;11(Suppl 4):S564-9.
BACKGROUND: Angelica shikokiana is a Japanese medicinal herb that is included among food and drug preparations protecting against cancer; however, there is no previous report about the cytotoxicity of A. shikokiana or its bioactive compounds. OBJECTIVE: This study was designed to investigate the cytotoxic activities of A. shikokiana methanol extract (AME) and its isolated compounds and to identify the molecular mechanisms of the cytotoxicity. MATERIALS AND METHODS: Cytotoxicity and selectivity was investigated by measuring the IC50 values on five cancer cell lines; human hepatocellular carcinoma, rhabdomyosarcoma (RD), colorectal carcinoma, human epithelioma and human breast adenocarcinoma and one normal cell line; human lung fibroblasts. The effects on tubulin polymerization and histone deacetylase 8 (HDAC8), were examined to determine the mechanism of cytotoxicity. Docking study was designed to examine the binding affinity to the target molecules. RESULTS: Methanol extract and some of its isolated coumarins and flavonoids showed potent, selective cytotoxicity against cancer cell lines. AME and all isolated compounds inhibited tubulin polymerization. Angelicin and kaempferol-3-O-rutinoside were the most active compounds. Phenolic compounds and furanocoumarins showed binding affinity to colchicine binding site rather than the vinblastine binding site of tubulin microtubules. On the other side, quercetin, kaempferol, luteolin, chlorogenic acid, and Methyl chlorogenate exhibited the strongest activity against HDAC8 and the highest affinity to trichostatin A binding site. CONCLUSION: These findings provide the first scientific evidence of the cytotoxicity of AME through inhibition of tubulin polymerization and HDAC8 activity through its coumarin and flavonoid content. SUMMARY: The present study provides for the first time a clue for the cytotoxic activities of the AME. Our results indicate that the cytotoxic activities are partially related to the ability of AME to inhibit tubulin polymerization and HDAC8 activity. Isolated compounds; Angelicin and kaempferol-3-O-rutinoside showed the strongest inhibition of tubulin polymerization through binding to colchicine binding domain of tubulin microtubules. Phenolic compounds; quercetin, luteolin, kaempferol, chlorogenic acid and Methyl chlorogenate exhibited a strong inhibition of HDAC8 through binding to TSA binding site. This, however, further detailed pharmacological and in vivo studies should be the next step in evaluating the cytotoxic activities of AME and its active compounds that are currently ongoing. Abbreviations used: AME: Methanol extract of the aerial part of A. shikokiana, HDACs: Histone deacetylases,HDAC8: Histone deacetylase 8.
[Chemical Composition of n-Butanol Fraction from Polygonum amplexicaule var. sinense].[Pubmed:26930981]
Zhong Yao Cai. 2015 Sep;38(9):1872-4.
OBJECTIVE: To study chemical composition of n-butanol fraction from Polygonoum amplexicaule var. sinense. METHODS: TLC,normal-phase silica gel, reveres-phase silica gel, Sephadex-LH and semi-preparative HPLC were used to isolate chemical compositions of n-butanol fraction from Polygonoum amplexicaule var. sinense. RESULTS: Nine compounds were identified as: caffeic acid n-butly ester (1), p-methoxy benzoic acid propyl ester (2),p-E-coumarin quinic acid methyl ester (3),p-Z-coumarin quinic acid methyl ester (4), ethyl ferulate (5), cinchonain I a (6), cinchonain Ib (7), Methyl chlorogenate(8), and 6-O-beta-D-caffeoylglucose (9). CONCLUSION: All compounds are isolated from this genus for the first time.
In vitro neuroprotective activities of compounds from Angelica shikokiana Makino.[Pubmed:25786165]
Molecules. 2015 Mar 16;20(3):4813-32.
Angelica shikokiana is widely marketed in Japan as a dietary food supplement. With a focus on neurodegenerative conditions such as Alzheimer's disease, the aerial part was extracted and through bio-guided fractionation, fifteen compounds [alpha-glutinol, beta-amyrin, kaempferol, luteolin, quercetin, kaempferol-3-O-glucoside, kaempferol-3-O-rutinoside, Methyl chlorogenate, chlorogenic acid, hyuganin E, 5-(hydroxymethyl)-2-furaldehyde, beta-sitosterol-3-O-glucoside, adenosine (isolated for the first time from A. shikokiana), isoepoxypteryxin and isopteryxin] were isolated. Isolated compounds were evaluated for in vitro neuroprotection using acetylcholine esterase inhibitory, protection against hydrogen peroxide and amyloid beta peptide (Abeta25-35)-induced neurotoxicity in neuro-2A cells, scavenging of hydroxyl radicals and intracellular reactive oxygen species and thioflavin T assays. Quercetin showed the strongest AChE inhibition (IC50 value = 35.5 microM) through binding to His-440 and Tyr-70 residues at the catalytic and anionic sites of acetylcholine esterase, respectively. Chlorogenic acid, its methyl ester, quercetin and luteolin could significantly protect neuro-2A cells against H2O2-induced neurotoxicity and scavenge hydroxyl radical and intracellular reactive oxygen species. Kaempferol-3-O-rutinoiside, hyuganin E and isoepoxypteryxin significantly decreased Abeta25-35-induced neurotoxicity and Th-T fluorescence. To the best of our knowledge, this is the first report about neuroprotection of hyuganin E and isoepoxypteryxin against Abeta25-35-induced neurotoxicity.
A new sesquiterpene lactone and other constituents of Moquiniastrum polymorphum subsp. floccosum (Asteraceae).[Pubmed:25532275]
Nat Prod Commun. 2014 Nov;9(11):1541-3.
A new guaianolide, 1S, 3S, 5R, 6S, 7S, 11R-l-hydroxy-11alpha,13-dihydrozaluzanin C (1), was isolated from Moquiniastrum polymorphum subsp. floccosum trunk bark, together with fifteen known compounds, which were identified as 11alpha,13-dihydroglucozaluzanin C (2), 8alpha-hydroxy-11alpha,13-dihydrozaluzanin C (3), zaluzanin C (4), gochnatiolide B (5), ethyl caffeate (6), Methyl chlorogenate (7), ethyl chlorogenate (8), methyl 3,5-dicaffeoyl quinate (9), ethyl 3,5-dicaffeoyl quinate (10), methyl 4,5-dicaffeoyl quinate (11), ethyl 4,5-dicaffeoyl quinate (12), ethyl 3,4-dicaffeoyl quinate (13), 3,5-dicaffeoyl quinic acid (14), 4,5-dicaffeoyl quinic acid (15), and 3,4-dicaffeoyl quinic acid (16). With the exception of 5, all known compounds are being reported for the first time in M. polymorphum.
[Chemical studies on roots of Ficus hirta].[Pubmed:24494557]
Zhongguo Zhong Yao Za Zhi. 2013 Nov;38(21):3696-701.
Seventeen compounds were isolated from the 95% ethanolic extract of the root of Ficus hirta. Their structures were identified on the basis of physicochemical properties and spectral data analysis. The structures were elucidated as cyclomorusin (1), 3-O-[(6-O-E-sinapoyl)-beta-D-glucopyranosyl]-(1 --> 2)-beta-D-glucopyranoside (2), 3,5,4'-trihydroxy-6,7,3'-trimethoxyflavone (3), quercetin (4), tricin (5), acacetin (6), luteolin (7), apigenin (8), (E) -suberenol (9), meranzin hydrate (10), methyl eugenol (11), 3-methoxy-4-hydroxybenzoic acid (12), p-hydroxybenzoic acid (13), Methyl chlorogenate (14), emodin (15), alpha-amyrin acetate (16), and beta-sitosterol emodin (17), respectively. Compounds 1-6, 9-15 were isolated from this plant for the first time.
Synthesis of 1-O-methylchlorogenic acid: reassignment of structure for MCGA3 isolated from bamboo (Phyllostachys edulis) leaves.[Pubmed:24460043]
J Agric Food Chem. 2014 Feb 26;62(8):1860-5.
The first synthesis of 1-O-methylchlorogenic acid is described. The short and efficient synthesis of this compound provides laboratory-scale quantities of the material to investigate its biological properties. The synthesis involves C-1 alkylation of the known (-)-4,5-cyclohexylidenequinic acid lactone followed by methoxide opening to the hydroxyl ester. Acylation of the C-5 hydroxyl group followed by sequential removal of protecting groups afforded 1-O-methylchlorogenic acid. The NMR spectroscopic characteristics of this compound do not coincide with those reported for the original isolation from bamboo (Phyllostachys edulis) leaves of the compound designated MCGA3. Comparison of the published spectroscopic data reported for MCGA3, with both reported literature values and spectroscopic data obtained from an authentic sample, leads to the conclusion that the compound isolated from bamboo (Phyllostachys edulis) leaves is instead Methyl chlorogenate.
[Chemical constituents of Neoalsomitra integrifoliola].[Pubmed:22993988]
Zhongguo Zhong Yao Za Zhi. 2012 Jun;37(11):1593-6.
OBJECTIVE: To study the chemical constituents of the n-BuOH fraction of 95% ethanolic extract of leaves of Neoalsomitra integrifoliola. METHOD: The compounds were isolated with kinds of column chromatography. The structures were determined by MS and NMR spectroscopic techniques. RESULT: Eight compounds were isolated from the n-BuOH fraction of 95% ethanolic extract and their structures were identified as 2-phenylethyl rutinoside (1), rutin (2), kaempferol-3-O-alpha-L-rhamnopyranosyl-(1-->6)-beta-D-glucopyranoside (3), isorhamnetin-3-O-alpha-L-rhamnopyranosyl-(1-->6)-beta-D-glucopyranoside (4), Methyl chlorogenate (5), guanosine (6), adenosine (7), myo-inositol (8), respectively. CONCLUSION: All compounds were isolated from this genus for the first time.
Phenolic constituents from the fruit juice of Flacourtia inermis.[Pubmed:21985676]
Nat Prod Res. 2012;26(3):278-81.
A chemical investigation of the fruit juice of Flacourtia inermis furnished five caffeoylquinic acid derivatives: Methyl chlorogenate (1), methyl 5-O-caffeoylquinate (2), methyl 4-O-caffeoylquinate (3), n-butyl chlorogenate (4), n-butyl 5-O-caffeoylquinate (5) and a rare phenolic glucoside (rel)-6alpha-benzoyloxy-1alpha,2alpha-dihydroxy-5-oxocyclohex-3-enecarboxylic acid 2-(6-O-benzoyl-beta-D-glucopyranosyloxy)-5-hydroxybenzyl ester (6), together with quinic acid (7) and malic acid (8). Compounds 1, 2, 4 and 5 showed strong radical scavenging properties towards the 2,2'-diphenyl-1-picrylhydrazyl radical.


