OstholCAS# 484-12-8 |
Quality Control & MSDS
Number of papers citing our products

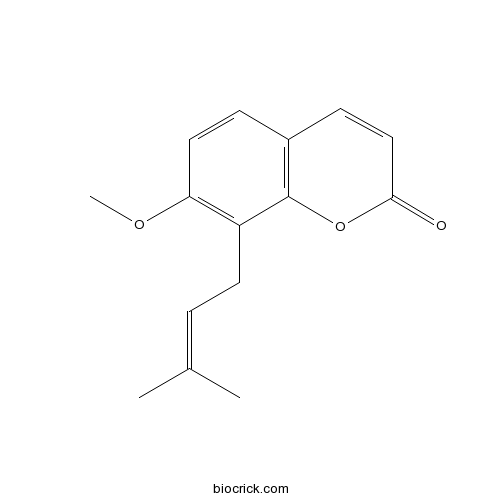
Chemical structure

3D structure
| Cas No. | 484-12-8 | SDF | Download SDF |
| PubChem ID | 10228 | Appearance | White powder |
| Formula | C15H16O3 | M.Wt | 244.3 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Synonyms | NSC 31868; Osthol; Ostol | ||
| Solubility | DMSO : 125 mg/mL (511.69 mM; Need ultrasonic and warming) | ||
| Chemical Name | 7-methoxy-8-(3-methylbut-2-enyl)chromen-2-one | ||
| SMILES | CC(=CCC1=C(C=CC2=C1OC(=O)C=C2)OC)C | ||
| Standard InChIKey | MBRLOUHOWLUMFF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H16O3/c1-10(2)4-7-12-13(17-3)8-5-11-6-9-14(16)18-15(11)12/h4-6,8-9H,7H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Osthol is a natural antihistamine alternative, may be a potential inhibitor of histamine H1 receptor activity. Osthol has toxicity, may be used as bio-pesticides. Osthol is an inhibitor of human Pgp and multidrug efflux pumps of Staphylococcus aureus , reversing the resistance against frontline antibacterial drugs.Osthol has anti-allergic, antiosteoporosis, anti-fatty liver, antitumor, and cardioprotective effects. Osthol inhibits hepatic SREBP-1c/2 mRNA expressions and subsequent modulation of SREBP-1c/2-mediated target genes such as FAS, CYP7A and LDL receptor; it can stimulate the osteoblastic differentiation of rat calvarial osteoblast cultures by the BMP-2/p38MAPK/Runx-2/osterix pathway. |
| Targets | PPAR | LDL | P450 (e.g. CYP17) | P-gp | p38MAPK | FAS | BMP-2 | Runx-2 | Histamine H1 receptor |
| In vitro | Acute toxicity assessment of Osthol content in bio-pesticides using two aquatic organisms.[Pubmed: 25518842]Environ Health Toxicol. 2014 Dec 10;29:e2014020.This study focused on the assessment of acute toxicity caused by Osthol, a major component of environment-friendly biological pesticides, by using two aquatic organisms.
Antitumor effects of Osthol from Cnidium monnieri: an in vitro and in vivo study.[Pubmed: 17154232 ]Phytother Res. 2007 Mar;21(3):226-30.Cnidium monnieri (L.) Cusson is a Chinese medicine which is used widely by traditional medicine doctors. Osthol is a major bio-activity compound of the herb.
|
| In vivo | Osthol attenuates hepatic steatosis via decreased triglyceride synthesis not by insulin resistance.[Pubmed: 25206279]World J Gastroenterol. 2014 Sep 7;20(33):11753-61.To evaluate the effects of Osthol on intrahepatic fat synthesis, β-oxidation, inflammation, and insulin resistance by multifaceted analysis.
Reduction of rat cardiac hypertrophy by osthol is related to regulation of cardiac oxidative stress and lipid metabolism.[Pubmed: 22918576]Lipids. 2012 Oct;47(10):987-94.The objective of this study was to examine the therapeutic effect of Osthol, a coumarin compound isolated from the fruit of Cnidium monnieri (L.) Cusson, on cardiac hypertrophy in rats and investigate its potential mechanisms.
|
| Kinase Assay | Osthol and curcumin as inhibitors of human Pgp and multidrug efflux pumps of Staphylococcus aureus: Reversing the resistance against frontline antibacterial drugs[Reference: WebLink]Med. Chem. Co., 2014, 5(10):1540-7.The in-house IIIM natural product repository of 302 small molecules was screened for their ability to inhibit p-glycoprotein (Pgp) in Pgp-overexpressing human adenocarcinoma LS-180 cells.
|
| Cell Research | Osthol, a coumarin isolated from common cnidium fruit, enhances the differentiation and maturation of osteoblasts in vitro.[Pubmed: 21734431]Click chemistry inspired synthesis and bioevaluation of novel triazolyl derivatives of osthol as potent cytotoxic agents.[Pubmed: 25062005]Eur J Med Chem. 2014 Sep 12;84:545-54.A new series of diverse triazoles linked through the hydroxyl group of lactone ring opened Osthol (1) were synthesized using click chemistry approach.
Pharmacology. 2011;88(1-2):33-43.The effect of Osthol on osteoblasts was investigated in primary osteoblastic cells isolated from newborn Wistar rats.
|
| Animal Research | Anti-allergic effects of cnidii monnieri fructus (dried fruits of Cnidium monnieri) and its major component, osthol.[Pubmed: 12081154]Osthol ameliorates fat milk-induced fatty liver in mice by regulation of hepatic sterol regulatory element-binding protein-1c/2-mediated target gene expression.[Pubmed: 21620823]Eur J Pharmacol. 2011 Sep;666(1-3):183-8.The objective of this study was to examine the therapeutic effect of Osthol, an active constituent of Cnidium monnieri (L.) Cusson (Apiaceae), in hyperlipidemic fatty liver mice and investigate the potential mechanism of the Osthol treatment.
Biol Pharm Bull. 2002 Jun;25(6):809-12.
|

Osthol Dilution Calculator

Osthol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0933 mL | 20.4666 mL | 40.9333 mL | 81.8666 mL | 102.3332 mL |
| 5 mM | 0.8187 mL | 4.0933 mL | 8.1867 mL | 16.3733 mL | 20.4666 mL |
| 10 mM | 0.4093 mL | 2.0467 mL | 4.0933 mL | 8.1867 mL | 10.2333 mL |
| 50 mM | 0.0819 mL | 0.4093 mL | 0.8187 mL | 1.6373 mL | 2.0467 mL |
| 100 mM | 0.0409 mL | 0.2047 mL | 0.4093 mL | 0.8187 mL | 1.0233 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Osthole is a natural antihistamine alternative. Osthole may be a potential inhibitor of histamine H1 receptor activity.
In Vitro:Osthole (p<0.0001) and Fexofenadine (p<0.001) inhibit increased HRH-1 mRNA expression induced by histamine in the study group. This result is also observed in cells cultured with histamine/Osthole; where combined substances decreased HRH-1 mRNA expression compared to histamine (p<0.0001)[1]. Assessment of cell viability does not detect obvious toxicity when Osthole is used at a dose up to 100 µM. However, when the dose reached 500 µM, Osthole started to show toxic effect. Based on these observations, Osthole is used in all in vitro studies at the dose range of 10 to 100 µM. Osthole dose-dependently promotes osteoblast differentiation, as shown by the upregulation of osteoblast differentiation marker genes such as type I collagen (col1), bone sialoprotein (BSP) and osteocalcin (OC) (2 days of culture). Osthole promotes ALP activity in mouse primary osteoblasts in a dose-dependent manner[2].
In Vivo:Subcutaneous injection of Osthole at a dose of 5 mg/kg per day onto mouse calvariae significantly stimulates local bone formation, as shown by histologic analysis of calvarial samples harvested 2 weeks after the last injection and stained with H&E; orange G. Histomorphometric analysis reveals that Osthole has a significant effect on bone formation as potent as the positive control, the microtubule inhibitor TN-16. This effect, however, is not seen when Osthole is used at a dose of 1 mg/kg per day. Intraperitoneal injection of Osthole for 8 weeks significantly reverses bone loss in the ovariectomized rats. Histologic examination of the L4samples stained with trinitrophenol poinsettia demonstrates a partial recovery of the trabecular structure in ovariectomized rats treated with Osthole. Histomorphometric analysis shows that treatment with Osthole significantly increases total BMD, trabecular bone volume, and trabecular thickness and decreases trabecular separation[2].
References:
[1]. Kordulewska NK, et al. Changes in gene expression induced by histamine, fexofenadine and osthole: Expression of histamine H1 receptor, COX-2, NF-κB, CCR1, chemokine CCL5/RANTES and interleukin-1β in PBMC allergic and non-allergic patients. Immunobiology.
[2]. Tang DZ, et al. Osthole stimulates osteoblast differentiation and bone formation by activation of beta-catenin-BMP signaling. J Bone Miner Res. 2010 Jun;25(6):1234-45.
[3]. Sun W, et al. Osthole pretreatment alleviates TNBS-induced colitis in mice via both cAMP/PKA-dependent and independent pathways. Acta Pharmacol Sin. 2017 Aug;38(8):1120-1128.
- Chrysophanol 1-glucoside
Catalog No.:BCC8146
CAS No.:4839-60-5
- N-Demethylloine
Catalog No.:BCN2004
CAS No.:4839-19-4
- N4-Benzoyl-2'-deoxycytidine
Catalog No.:BCC9071
CAS No.:4836-13-9
- Purmorphamine
Catalog No.:BCC3641
CAS No.:483367-10-8
- SB-674042
Catalog No.:BCC1931
CAS No.:483313-22-0
- Luvangetin
Catalog No.:BCN7527
CAS No.:483-92-1
- Calycanthoside
Catalog No.:BCN5580
CAS No.:483-91-0
- Toddalolactone
Catalog No.:BCN2393
CAS No.:483-90-9
- Sphondin
Catalog No.:BCN5579
CAS No.:483-66-9
- Cheilanthifoline
Catalog No.:BCN7827
CAS No.:483-44-3
- (-)-Isocorypalmine
Catalog No.:BCN2723
CAS No.:483-34-1
- Cephaeline
Catalog No.:BCC8143
CAS No.:483-17-0
- Osthenol
Catalog No.:BCN8342
CAS No.:484-14-0
- 9-Phenanthrol
Catalog No.:BCC7989
CAS No.:484-17-3
- Bergapten
Catalog No.:BCN5582
CAS No.:484-20-8
- Dictamnine
Catalog No.:BCN1273
CAS No.:484-29-7
- Angiotensin I (human, mouse, rat)
Catalog No.:BCC1004
CAS No.:484-42-4
- Isodictamnine
Catalog No.:BCN7066
CAS No.:484-74-2
- Okanin
Catalog No.:BCN6475
CAS No.:484-76-4
- DPH
Catalog No.:BCC1538
CAS No.:484049-04-9
- Brucine sulfate
Catalog No.:BCN2460
CAS No.:4845-99-2
- ProTx I
Catalog No.:BCC6255
CAS No.:484598-35-8
- ProTx II
Catalog No.:BCC6103
CAS No.:484598-36-9
- N-Nornuciferine
Catalog No.:BCN4048
CAS No.:4846-19-9
Antitumor effects of Osthol from Cnidium monnieri: an in vitro and in vivo study.[Pubmed:17154232]
Phytother Res. 2007 Mar;21(3):226-30.
Cnidium monnieri (L.) Cusson is a Chinese medicine which is used widely by traditional medicine doctors. Osthol is a major bio-activity compound of the herb. In this study, Osthol was isolated from C. monnieri and its in vitro and in vivo antitumor effects studied. The results of the in vitro study showed: that Osthol inhibited the growth of HeLa, in a time- and concentration-dependent manner, with IC(50) values of 77.96 and 64.94 microm for 24 and 48 h, respectively; that Osthol had lower cytotoxic effects in primary cultured normal cervical fibroblasts; and that increased DNA fragmentation and activated PARP in HeLa after treatment with Osthol which could induce apoptosis. The results of the in vivo model showed that the survival days of the P-388 D1 tumor-bearing CDF(1) mice were prolonged (ILS% = 37) after Osthol (30 mg/kg) was given once a day for 9 days. Based on these results, it is suggested that Osthol could inhibit P-388 D1 cells in vivo and induce apoptosis in HeLa cells in vitro, and that Osthol is good lead compound for developing antitumor drugs. However, C. formosanum Yabe of Taiwan's endemic plants contained little Osthol, with no imperatorin, and its major components were different from that of C. monnieri. Therefore, it is suggested that C. formosanum also may possess economic worth.
Acute toxicity assessment of Osthol content in bio-pesticides using two aquatic organisms.[Pubmed:25518842]
Environ Health Toxicol. 2014 Dec 10;29:e2014020.
OBJECTIVES: This study focused on the assessment of acute toxicity caused by Osthol, a major component of environment-friendly biological pesticides, by using two aquatic organisms. METHODS: The assessment of acute toxicity caused by Osthol was conducted in Daphnia magna and by examining the morphological abnormalities in Danio rerio embryos. RESULTS: The median effective concentration value of Osthol in D. magna 48 hours after inoculation was 19.3 muM. The median lethal concentration of D. rerio embryo at 96 hours was 30.6 muM. No observed effect concentration and predicted no effect concentration values of Osthol in D. magna and D. rerio were calculated as 5.4 and 0.19 muM, respectively. There was an increase in the morphological abnormalities in D. rerio embryo due to Osthol over time. Coagulation, delayed hatching, yolk sac edema, pericardial edema, and pigmentation were observed in embryos at 24-48 hours. Symptoms of scoliosis and head edema occurred after 72 hours. In addition, bent tails, ocular defects, and symptoms of collapse were observed in fertilized embryo tissue within 96 hours. Ocular defects and pigmentation were the additional symptoms observed in this study. CONCLUSIONS: Because Osthol showed considerable toxicity levels continuous toxicity evaluation in agro-ecosystems is necessary when bio-pesticides containing Osthol are used.
Click chemistry inspired synthesis and bioevaluation of novel triazolyl derivatives of osthol as potent cytotoxic agents.[Pubmed:25062005]
Eur J Med Chem. 2014 Sep 12;84:545-54.
A new series of diverse triazoles linked through the hydroxyl group of lactone ring opened Osthol (1) were synthesized using click chemistry approach. All the derivatives were subjected to 3-(4,5-Dimethylthiazol-yl)-diphenyl tetrazoliumbromide (MTT) cytotoxicity screening against a panel of seven different human cancer cell lines viz. colon (colo-205), colon (HCT-116), breast (T47D), lung (NCI-H322), lung (A549), prostate (PC-3) and Skin (A-431) to check their cytotoxic potential. Interestingly, among the tested molecules, most of the analogs displayed better cytotoxic activity than the parent Osthol (1). Of the synthesized triazoles, compounds 8 showed the best activity with IC50 of 1.3, 4.9, 3.6, 41.0, 35.2, 26.4 and 7.2 muM against colon (Colo-205 and HCT-116), breast (T47D), lung (NCI-H322 and A549), prostate (PC-3) and Skin (A-431) cancer lines respectively. Compound 8 induced potent apoptotic effects in Colo-205 cells. The population of apoptotic cells increased from 11.4% in case of negative control to 24.1% at 25 muM of 8. Compound 8 also induced a remarkable decrease in mitochondrial membrane potential (LambdaPsim) leading to apoptosis of cancer cells used. The present study resulted in identification of broad spectrum cytotoxic activity of analogs bearing electron withdrawing substituents, besides the enhanced selective activity of analogs with electron donating moieties.
Anti-allergic effects of cnidii monnieri fructus (dried fruits of Cnidium monnieri) and its major component, osthol.[Pubmed:12081154]
Biol Pharm Bull. 2002 Jun;25(6):809-12.
Anti-allergic effects (types I and IV) of the 70% ethanol extract (CM-ext) obtained from Cnidii Monnieri Fructus (dried fruits of Cnidium monnieri) were investigated on 48 h homologous passive cutaneous anaphylaxis (PCA), 2, 4-dinitrofluorobenzene (DNFB)-induced contact dermatitis and picryl chloride (PC)-induced contact dermatitis in experimental animals. CM-ext showed inhibitory effects on these allergic models. Osthol isolated from CM-ext also had the inhibitory effects. These results suggested that Cnidii Monnieri Fructus might be useful as an agent for allergic diseases and that its anti-allergic effect was partially attributable to a coumarin derivative, Osthol.
Osthol, a coumarin isolated from common cnidium fruit, enhances the differentiation and maturation of osteoblasts in vitro.[Pubmed:21734431]
Pharmacology. 2011;88(1-2):33-43.
The effect of Osthol on osteoblasts was investigated in primary osteoblastic cells isolated from newborn Wistar rats. Osthol was supplemented into cultured medium at 10(-)(7), 10(-)(6), 10(-)(5) and 10(-)(4) mol/l, respectively. No stimulating effect was found on cell proliferation, but 10(-)(5) mol/l Osthol caused a significant increase in alkaline phosphatase (ALP) activity. Osteogenic differentiation markers were examined over a period of time at this concentration, and compared with control cells that were not supplemented with Osthol. The results showed that the ALP activity, osteocalcin secretion and calcium deposition level in cells treated with Osthol were 1.52, 2.74 and 2.0 times higher, respectively, than in the control cells. Results of ALP histochemical staining and mineralized bone nodule assays both showed that the number and area achieved in Osthol-treated cells were 1.53-fold higher than in control cells. The gene expression of the growth and transcription factors basic fibroblast growth factor, insulin-like growth factor I, bone morphogenetic protein 2 (BMP-2), runt-related gene 2 (Runx-2) and osterix, which are associated with bone development, were also investigated. The increase in mRNA expression was 1.94, 1.74, 1.68, 1.83 and 2.31 times, respectively, higher compared to the control. Furthermore, Osthol increased the protein expression of p38 mitogen-activated protein kinase (MAPK) and type I collagen. p38MAPK protein and collagen in Osthol-treated cells were 1.42 and 1.58 times higher in Osthol-treated cells compared to the control. The results of these studies support the conclusion that Osthol significantly enhances the osteogenic differentiation of cultured osteoblasts. The results also indicated that Osthol could stimulate the osteoblastic differentiation of rat calvarial osteoblast cultures by the BMP-2/p38MAPK/Runx-2/osterix pathway and that Osthol may be used as an important compound in the development of new antiosteoporosis drugs.
Reduction of rat cardiac hypertrophy by osthol is related to regulation of cardiac oxidative stress and lipid metabolism.[Pubmed:22918576]
Lipids. 2012 Oct;47(10):987-94.
The objective of this study was to examine the therapeutic effect of Osthol, a coumarin compound isolated from the fruit of Cnidium monnieri (L.) Cusson, on cardiac hypertrophy in rats and investigate its potential mechanisms. The rats with cardiac hypertrophy induced by renovascular hypertension were given Osthol orally by gavage for 4 weeks. The results showed that in the Osthol 20 mg/kg group, the blood pressure, heart weight index and myocardial malondialdehyde content were lowered (p < 0.001, p = 0.002 and p = 0.025, respectively), the myocardial superoxide dismutase and glutathione peroxidase contents were increased (p < 0.001), and the elevated unesterified fatty acids and triacylglycerols in myocardial tissues were decreased (p = 0.017 and p = 0.004, respectively). At the same time, the myocardial peroxisome proliferator-activated receptor (PPAR)-alpha and carnitine palmitoyltransferase (CPT)-1a mRNA expressions were increased and the myocardial diacylglycerol acyltransferase (DGAT) mRNA expression was decreased in the Osthol 20 mg/kg group (p < 0.001). Osthol treatment was associated with a decreased cross-sectional area of cardiomyocytes (p < 0.001). These findings suggest that Osthol may exert a therapeutic effect on cardiac hypertrophy in rats, and its mechanisms may be related to the improvement of myocardial oxidative stress and lipid metabolism via regulation of PPARalpha-mediated target gene expressions including an increase in CPT-1a mRNA expression and a decrease in DGAT mRNA expression.
Osthol attenuates hepatic steatosis via decreased triglyceride synthesis not by insulin resistance.[Pubmed:25206279]
World J Gastroenterol. 2014 Sep 7;20(33):11753-61.
AIM: To evaluate the effects of Osthol on intrahepatic fat synthesis, beta-oxidation, inflammation, and insulin resistance by multifaceted analysis. METHODS: Sprague-Dawley rats (n = 30) were randomly divided into control, non-alcoholic fatty liver disease (NAFLD), and Osthol groups. NAFLD and Osthol groups were fed with a high-fat diet for 14 wk. After 8 wk of the high-fat diet, the Osthol group also received Osthol 20 mg/kg orally 5 times/wk. To assess the insulin resistance, oral glucose tolerance was performed at the end of 14 wk. Immunohistochemical (4-HNE, F4/80) and hematoxylin and eosin (HE) staining were performed on liver tissue extracts after animal sacrifice at 14 wk. SREBP1c, FAS, SCD-1, PPAR-alpha, CROT, MCP-1, IRS-1, and IRS-2 mRNA expressions were assessed with reverse transcription-polymerase chain reaction. RESULTS: HE staining revealed that, compared with the NAFLD group, the Osthol group showed significantly decreased intrahepatic fat content (39.4% vs 21.0%; P = 0.021). SREBP1c expression in the NAFLD group increased compared to controls (P = 0.0001), while Osthol treatment decreased SREBP1c expression compared with the NAFLD group (P = 0.0059). In the Osthol group, intrahepatic FAS and SCD-1, which act downstream of SREBP1c, decreased significantly compared with the NAFLD group. Moreover, PPAR-alpha expression in the Osthol group was also significantly higher than in the NAFLD group (P = 0.0147). CONCLUSION: Osthol treatment attenuated liver steatosis by decreasing de novo liver triglyceride synthesis and had nominal effects on insulin resistance and liver inflammation.
Osthol ameliorates fat milk-induced fatty liver in mice by regulation of hepatic sterol regulatory element-binding protein-1c/2-mediated target gene expression.[Pubmed:21620823]
Eur J Pharmacol. 2011 Sep;666(1-3):183-8.
The objective of this study was to examine the therapeutic effect of Osthol, an active constituent of Cnidium monnieri (L.) Cusson (Apiaceae), in hyperlipidemic fatty liver mice and investigate the potential mechanism of the Osthol treatment. A mouse model with hyperlipidemic fatty liver was induced by orally feeding the fat milk for 4 weeks. The experimental mice were then treated with Osthol 10-40 mg/kg for 6 weeks. After oral administration, the mice in the model and medicine-treated groups were continuously given the fat milk for 2 weeks again. Whereafter, the lipid levels in serum and liver, hepatic weight coefficient and histopathological evaluation were measured. The sterol regulatory element-binding protein (SREBP)-1c, SREBP-2, fatty acid synthase (FAS), low density lipoprotein (LDL) receptor and cholesterol 7alpha-hydroxylase (CYP7A) mRNA expressions in liver were examined. The results showed that in the Osthol-treated groups, the total cholesterol, triglyceride and free fatty acid levels in serum and liver, and the hepatic weight coefficient were gradually decreased with dose. Importantly, the histopathological evaluation of liver specimens demonstrated that Osthol might decrease lipid accumulation. Osthol could increase the mRNA expression of CYP7A and decrease the mRNA expressions of SREBP-1c, SREBP-2, FAS and LDL receptor in liver in fat milk-induced fatty liver mice. These results suggested that Osthol might exert the therapeutic effect on fat milk-induced fatty liver in mice, by inhibiting hepatic SREBP-1c/2 mRNA expressions and subsequent modulation of SREBP-1c/2-mediated target genes such as FAS, CYP7A and LDL receptor.


