OsthenolCAS# 484-14-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 484-14-0 | SDF | Download SDF |
| PubChem ID | 5320318 | Appearance | Powder |
| Formula | C14H14O3 | M.Wt | 230.26 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
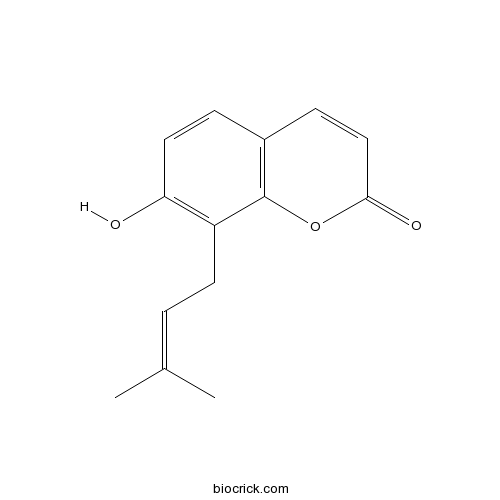
| Chemical Name | 7-hydroxy-8-(3-methylbut-2-enyl)chromen-2-one | ||
| SMILES | CC(=CCC1=C(C=CC2=C1OC(=O)C=C2)O)C | ||
| Standard InChIKey | RAKJVIPCCGXHHS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H14O3/c1-9(2)3-6-11-12(15)7-4-10-5-8-13(16)17-14(10)11/h3-5,7-8,15H,6H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Osthenol shows antitumor-promoting activity, it also has antifungal and antibacterial activities.Osthenol potently and selectively inhibited recombinant human monoamine oxidase-A (hMAO-A) with an IC50 value of 0.74 µM; it exhibited a highly potent inhibitory activity on 5alpha-reductase type I in LNCaP cells with an IC50 value of 0.1 microg/ml; it also exhibited inhibitory activity on COX-1 with the IC50 value of 64.3 microM. |
| Targets | Antifection | MAO-A | 5alpha-reductase | COX-1 |
| In vitro | Studies on coumarins from fruit of Cnidium monnieri and their cytotoxic activities.[Pubmed: 26983206]Zhongguo Zhong Yao Za Zhi. 2015 Sep;40(18):3594-7.This study is to study is to investigate the coumarins from Fruit of Cnidium monnieri and their cytotoxic activities. Antifungal activity of coumarins.[Pubmed: 18386483 ]Z Naturforsch C. 2008 Jan-Feb;63(1-2):21-8.
Antibacterial activity of coumarins.[Pubmed: 16320610 ]Z Naturforsch C. 2005 Sep-Oct;60(9-10):693-700.
|
| Kinase Assay | Inhibitory effects of Angelica pubescens f. biserrata on 5-lipoxygenase and cyclooxygenase.[Pubmed: 9741298 ]Inhibitors of 5alpha -reductase type I in LNCaP cells from the roots of Angelica koreana.[Pubmed: 11859469]Osthenol, a prenylated coumarin, as a monoamine oxidase A inhibitor with high selectivity.[Pubmed: 30686752]Bioorg Med Chem Lett. 2019 Mar 15;29(6):839-843.
Planta Med. 2002 Feb;68(2):162-3.
Planta Med. 1998 Aug;64(6):525-9.
|
| Cell Research | Antitumor-promoting activity of coumarins from citrus plants.[Pubmed: 15678381]Planta Med. 2005 Jan;71(1):84-7.
|
| Structure Identification | Zhongguo Zhong Yao Za Zhi. 2017 Jun;42(11):2102-2109.Chemical constituents from lipophilic parts in roots of Angelica dahurica cv.Yubaizhi.[Pubmed: 28822155 ]The chemical constituents from lipophilic parts in the roots of Angelica dahurica cv. Yubaizhi were studied in this paper.
|

Osthenol Dilution Calculator

Osthenol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3429 mL | 21.7146 mL | 43.4292 mL | 86.8583 mL | 108.5729 mL |
| 5 mM | 0.8686 mL | 4.3429 mL | 8.6858 mL | 17.3717 mL | 21.7146 mL |
| 10 mM | 0.4343 mL | 2.1715 mL | 4.3429 mL | 8.6858 mL | 10.8573 mL |
| 50 mM | 0.0869 mL | 0.4343 mL | 0.8686 mL | 1.7372 mL | 2.1715 mL |
| 100 mM | 0.0434 mL | 0.2171 mL | 0.4343 mL | 0.8686 mL | 1.0857 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Osthol
Catalog No.:BCN5581
CAS No.:484-12-8
- Chrysophanol 1-glucoside
Catalog No.:BCC8146
CAS No.:4839-60-5
- N-Demethylloine
Catalog No.:BCN2004
CAS No.:4839-19-4
- N4-Benzoyl-2'-deoxycytidine
Catalog No.:BCC9071
CAS No.:4836-13-9
- Purmorphamine
Catalog No.:BCC3641
CAS No.:483367-10-8
- SB-674042
Catalog No.:BCC1931
CAS No.:483313-22-0
- Luvangetin
Catalog No.:BCN7527
CAS No.:483-92-1
- Calycanthoside
Catalog No.:BCN5580
CAS No.:483-91-0
- Toddalolactone
Catalog No.:BCN2393
CAS No.:483-90-9
- Sphondin
Catalog No.:BCN5579
CAS No.:483-66-9
- Cheilanthifoline
Catalog No.:BCN7827
CAS No.:483-44-3
- (-)-Isocorypalmine
Catalog No.:BCN2723
CAS No.:483-34-1
- 9-Phenanthrol
Catalog No.:BCC7989
CAS No.:484-17-3
- Bergapten
Catalog No.:BCN5582
CAS No.:484-20-8
- Dictamnine
Catalog No.:BCN1273
CAS No.:484-29-7
- Angiotensin I (human, mouse, rat)
Catalog No.:BCC1004
CAS No.:484-42-4
- Isodictamnine
Catalog No.:BCN7066
CAS No.:484-74-2
- Okanin
Catalog No.:BCN6475
CAS No.:484-76-4
- DPH
Catalog No.:BCC1538
CAS No.:484049-04-9
- Brucine sulfate
Catalog No.:BCN2460
CAS No.:4845-99-2
- ProTx I
Catalog No.:BCC6255
CAS No.:484598-35-8
- ProTx II
Catalog No.:BCC6103
CAS No.:484598-36-9
- N-Nornuciferine
Catalog No.:BCN4048
CAS No.:4846-19-9
- Aristolochic acid C
Catalog No.:BCN2658
CAS No.:4849-90-5
Isolation and identification of metabolites of osthole in rats.[Pubmed:22630788]
Xenobiotica. 2012 Nov;42(11):1120-7.
Osthole (Ost), one of the major components of Cnidium monnieri (L.) Cusson, is had the structure of an isopentenoxy-coumarin with a range of pharmacological activities. In the present study, the metabolism of Ost in male Sprague-Dawley rats was investigated by identifying Ost metabolites excreted in rat urine. Following an oral dose of 40 mg/kg Ost, 10 phase I and 3 phase II metabolites were isolated from the urine of rats, and their structures identified on the basis of a range of spectroscopic data, including 2D-NMR techniques. These metabolites were fully characterized as 5'-hydroxyl-osthole (M-1), Osthenol (M-2), 4'-hydroxyl-osthole (M-3), 3, 5'-dihydroxyl-osthole (M-4), 5'-hydroxyl-Osthenol (M-5), 4'-hydroxyl-2', 3'-dihydro-Osthenol (M-6), 4'-hydroxyl-Osthenol (M-7), 3, 4'-dihydroxyl-osthole (M-8), 2', 3'-dihydroxyl-osthole (M-9), 5'-hydroxyl-2', 3'-dihydroosthole (M-10), Osthenol-7-O-beta-D-glucuronide (M-11), osthole-4'-O-beta-D-glucuronide (M-12) and osthole-5'-O-beta-D-glycuronate (M-13). This is the first identification of M-1, M-3 to M-13 in vivo. On the basis of the metabolites profile, a possible metabolic pathway for Ost metabolism in rats has been proposed. This is the first systematic study on the phases I and II metabolites of 8-isopentenoxy-coumarin derivative.
Molecular evolution of parsnip (Pastinaca sativa) membrane-bound prenyltransferases for linear and/or angular furanocoumarin biosynthesis.[Pubmed:26918393]
New Phytol. 2016 Jul;211(1):332-44.
In Apiaceae, furanocoumarins (FCs) are plant defence compounds that are present as linear or angular isomers. Angular isomers appeared during plant evolution as a protective response to herbivores that are resistant to linear molecules. Isomeric biosynthesis occurs through prenylation at the C6 or C8 position of umbelliferone. Here, we report cloning and functional characterization of two different prenyltransferases, Pastinaca sativa prenyltransferase 1 and 2 (PsPT1 and PsPT2), that are involved in these crucial reactions. Both enzymes are targeted to plastids and synthesize Osthenol and demethylsuberosin (DMS) using exclusively umbelliferone and dimethylallylpyrophosphate (DMAPP) as substrates. Enzymatic characterization using heterologously expressed proteins demonstrated that PsPT1 is specialized for the synthesis of the linear form, demethylsuberosin, whereas PsPT2 more efficiently catalyses the synthesis of its angular counterpart, Osthenol. These results are the first example of a complementary prenyltransferase pair from a single plant species that is involved in synthesizing defensive compounds. This study also provides a better understanding of the molecular mechanisms governing the angular FC biosynthetic pathway in apiaceous plants, which involves two paralogous enzymes that share the same phylogenetic origin.
A coumarin-specific prenyltransferase catalyzes the crucial biosynthetic reaction for furanocoumarin formation in parsley.[Pubmed:24354545]
Plant J. 2014 Feb;77(4):627-38.
Furanocoumarins constitute a sub-family of coumarin compounds with important defense properties against pathogens and insects, as well as allelopathic functions in plants. Furanocoumarins are divided into two sub-groups according to the alignment of the furan ring with the lactone structure: linear psoralen and angular angelicin derivatives. Determination of furanocoumarin type is based on the prenylation position of the common precursor of all furanocoumarins, umbelliferone, at C6 or C8, which gives rise to the psoralen or angelicin derivatives, respectively. Here, we identified a membrane-bound prenyltransferase PcPT from parsley (Petroselinum crispum), and characterized the properties of the gene product. PcPT expression in various parsley tissues is increased by UV irradiation, with a concomitant increase in furanocoumarin production. This enzyme has strict substrate specificity towards umbelliferone and dimethylallyl diphosphate, and a strong preference for the C6 position of the prenylated product (demethylsuberosin), leading to linear furanocoumarins. The C8-prenylated derivative (Osthenol) is also formed, but to a much lesser extent. The PcPT protein is targeted to the plastids in planta. Introduction of this PcPT into the coumarin-producing plant Ruta graveolens showed increased consumption of endogenous umbelliferone. Expression of PcPT and a 4-coumaroyl CoA 2'-hydroxylase gene in Nicotiana benthamiana, which does not produce furanocoumarins, resulted in formation of demethylsuberosin, indicating that furanocoumarin production may be reconstructed by a metabolic engineering approach. The results demonstrate that a single prenyltransferase, such as PcPT, opens the pathway to linear furanocoumarins in parsley, but may also catalyze the synthesis of Osthenol, the first intermediate committed to the angular furanocoumarin pathway, in other plants.
[Chemical constituents from lipophilic parts in roots of Angelica dahurica var. formosana cv. Chuanbaizhi].[Pubmed:26552172]
Zhongguo Zhong Yao Za Zhi. 2015 Jun;40(11):2148-56.
The chemical constituents from lipophilic parts in the roots of Angelica dahurica var. formosana cv. Chuanbaizhi were studied in this paper. The compounds were separated and purified by repeated column chromatographic methods on silica gel and HPLC, and the chemical structures of compounds were determined by spectral data analyses. Twenty-nine compounds were obtained and identified as isoimperatorin (1), beta-sitosterol (2), imperatorin (3), bergapten (4), Osthenol (5), xanthotoxin (6), isoimpinellin (7), dehydrogeijerin (8), phellopterin (9), isodemethylfuropinarine (10), 7-demethylsuberosin (11), alloimperatorin (12), xanthotoxol (13), isooxypeucedanin (14), alloisoimperatorin (15), demethylfuropinarine (16), 5-hydroxy-8-methoxypsoralen (17), oxypeucedanin methanolate (18), pabulenol (19), byakangelicin (20), marmesin (21), (+) -decursinol (22), heraclenol (23), oxypeucedanin hydrate (24), marmesinin (25), ulopterol (26), erythro-guaiacylglycerol-beta-ferulic acid ether (27), threo-guaiacylglycerol-beta-ferulic acid ether (28), and uracil (29). Compounds 5, 8, 11, 18, 21-23, and 26-28 were obtained from the roots of title plant for the first time.
[Chemical constituents from lipophilic parts in roots of Angelica dahurica cv.Yubaizhi].[Pubmed:28822155]
Zhongguo Zhong Yao Za Zhi. 2017 Jun;42(11):2102-2109.
The chemical constituents from lipophilic parts in the roots of Angelica dahurica cv. Yubaizhi were studied in this paper. The compounds were separated and purified by repeated column chromatographic methods on silica gel and HPLC, and the chemical structures of compounds were determined by spectral data analyses. Thirty-three compounds were obtained and identified as isoimperatorin (1), imperatorin (2), stigmasterol (3), isooxypeucedanin (4), pabulenol (5), psoralen (6), bergapten (7), isodemethylfuropinarine (8), phellopterin (9), Osthenol (10), alloimperatorin (11), xanthotoxin (12), xanthotoxol (13), isopimpinellin (14), alloisoimperatorin (15), beta-sitosterol (16), oxyalloimperatorin (17), pabularinone (18), 5-hydroxy-8-methoxypsoralen (19), columbianetin (20), heracol (21), isogosferol (22), 2''R-neobyakangelicol (23), byakangelicin ethoxide (24), byakangelicin (25), oxypeucedanin hydrate (26), uracil (27), umbelliferone (28), bergaptol (29), demethylfuropinarine (30), isobyakangelicol (31), oxypeucedanin ethanolate (32), heraclenol (33). Among them, compounds 8, 10, 17, 21, and 30 were obtained from the roots of title plant for the first time.
[Studies on coumarins from fruit of Cnidium monnieri and their cytotoxic activities].[Pubmed:26983206]
Zhongguo Zhong Yao Za Zhi. 2015 Sep;40(18):3594-7.
This study is to study is to investigate the coumarins from Fruit of Cnidium monnieri and their cytotoxic activities. The constituents were separated by column chromatography, and their structures were elucidated by spectroscopic data analyses. The isolated compounds were evaluated for their cytoxic activities by MTT method. Eleven compounds were isolated and identified as osthole (1), bergaptan (2), xanthotoxol (3), xanthotoxin (4), imperatorin (5), isopimpinellin (6), Osthenol (7), psoralen (8), 5,7-dimethoxycoumarin (9), oxypeucedaninhydrate (10), and swietenocoumarin F (11). Compounds 7, 9-11 were isolated from the Cnidium genus for the first time. Compounds 1,5,10 and 11 showed significant cytotoxic activities against L1210 cell lines at a concentration of 1 x 10(-5) mol x L(-1) with inhibitory rates of were 70.13, 63.10, 55.77, and 75.08% respectively.
Antifungal activity of coumarins.[Pubmed:18386483]
Z Naturforsch C. 2008 Jan-Feb;63(1-2):21-8.
The antifungal activity of 40 coumarins was tested against the fungal strains: Candida albicans (ATCC 14053), Aspergillus fumigatus (ATCC 16913) and Fusarium solani (ATCC 36031), using the broth microdilution method. Osthenol showed the most effective antifungal activity among all the compounds tested, with a MIC value of 125 microg/ml for Fusarium solani and 250 micro/ml for Candida albicans and Aspergillus fumigatus. The antifungal potential of this prenylated coumarin can be related to the presence of an alkyl group at C-8 position.


