Omaveloxolone (RTA-408)CAS# 1474034-05-3 |
- Cefditoren Pivoxil
Catalog No.:BCC4898
CAS No.:117467-28-4
- Balofloxacin
Catalog No.:BCC4892
CAS No.:127294-70-6
- Pefloxacin Mesylate Dihydrate
Catalog No.:BCC5089
CAS No.:149676-40-4
- Tinidazole
Catalog No.:BCC4866
CAS No.:19387-91-8
- Toltrazuril
Catalog No.:BCC4870
CAS No.:69004-03-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1474034-05-3 | SDF | Download SDF |
| PubChem ID | 71811910 | Appearance | Powder |
| Formula | C33H44F2N2O3 | M.Wt | 554.71 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Omaveloxolone | ||
| Solubility | DMSO : ≥ 100 mg/mL (180.27 mM) *"≥" means soluble, but saturation unknown. | ||
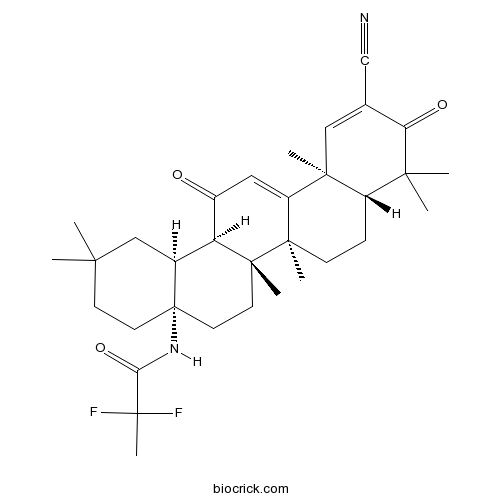
| Chemical Name | N-[(4aS,6aR,6bS,8aR,12aS,14aR,14bS)-11-cyano-2,2,6a,6b,9,9,12a-heptamethyl-10,14-dioxo-1,3,4,5,6,7,8,8a,14a,14b-decahydropicen-4a-yl]-2,2-difluoropropanamide | ||
| SMILES | CC1(CCC2(CCC3(C(C2C1)C(=O)C=C4C3(CCC5C4(C=C(C(=O)C5(C)C)C#N)C)C)C)NC(=O)C(C)(F)F)C | ||
| Standard InChIKey | RJCWBNBKOKFWNY-IDPLTSGASA-N | ||
| Standard InChI | InChI=1S/C33H44F2N2O3/c1-27(2)11-13-33(37-26(40)32(8,34)35)14-12-31(7)24(20(33)17-27)21(38)15-23-29(5)16-19(18-36)25(39)28(3,4)22(29)9-10-30(23,31)6/h15-16,20,22,24H,9-14,17H2,1-8H3,(H,37,40)/t20-,22-,24-,29-,30+,31+,33-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | RTA-408 is an antioxidant inflammation modulator (AIM), which activates Nrf2 and suppresses nitric oxide (NO).In Vitro:To evaluate the anti-inflammatory activity of RTA-408, RAW 264.7 mouse macrophage cells are treated with RTA-408 for two hours and then IFNγ is added to stimulate NO production and release into the media. RTA-408 dose-dependently reduces NO concentrations in the media with an IC50 value of 4.4±1.8 nM. The potency of RTA-408 in this assay is similar to that of Bardoxolone methyl, which has an IC50 value of 1.9±0.8 nM. Nrf2 activation is required for AIM-mediated NO suppression. A decrease in nitric oxide synthase 2 (Nos2) protein levels is observed in bardoxolone methyl-treated RAW 264.7 cells, which is attenuated when Nrf2 mRNA levels are reduced by siRNA. To evaluate the anticancer activity of RTA-408, a panel of eight human cell lines derived from tumors of different origin are treated with RTA-408 and measured cell growth 72 hours later using the sulforhodamine B (SRB) assay. RTA-408 inhibits the growth of all tumor lines with an average GI50 value of 260±74 nM. To determine whether RTA-408 induces apoptosis, the panel of tumor cells are treated with RTA-408 and the caspase substrate, DEVD-AFC, for 24 hours. RTA-408 dose-dependently increases DEVD-AFC cleavage, indicating that RTA-408 treatment triggers caspase activation in cancer cells. Caspase-3 and caspase-9 cleavage is also observed by western blot at the same concentrations of RTA-408 that increases DEVD-AFC cleavage[1].In Vivo:To determine whether RTA-408 is an effective mitigator of hematopoietic acute radiation syndrome after bone marrow-lethal doses of total-body irradiation (TBI), mice are administered 3 daily injections of 17.5 mg/kg RTA-408 beginning 24 h after TBI. Teatment with RTA-408 results in the 35 day survival of 100% of 7 Gy (LD40/35) TBI mice (P<0.05) and 60% of 7.5 Gy (LD100/13) TBI mice (P<0.0001)[2]. References: | |||||

Omaveloxolone (RTA-408) Dilution Calculator

Omaveloxolone (RTA-408) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8027 mL | 9.0137 mL | 18.0274 mL | 36.0549 mL | 45.0686 mL |
| 5 mM | 0.3605 mL | 1.8027 mL | 3.6055 mL | 7.211 mL | 9.0137 mL |
| 10 mM | 0.1803 mL | 0.9014 mL | 1.8027 mL | 3.6055 mL | 4.5069 mL |
| 50 mM | 0.0361 mL | 0.1803 mL | 0.3605 mL | 0.7211 mL | 0.9014 mL |
| 100 mM | 0.018 mL | 0.0901 mL | 0.1803 mL | 0.3605 mL | 0.4507 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Omaveloxolone (RTA-408)
- Azilsartan
Catalog No.:BCC5014
CAS No.:147403-03-0
- Ligupurpuroside B
Catalog No.:BCC8199
CAS No.:147396-02-9
- Ligupurpuroside A
Catalog No.:BCC8198
CAS No.:147396-01-8
- MKT 077
Catalog No.:BCC6241
CAS No.:147366-41-4
- Cylindramide
Catalog No.:BCN1832
CAS No.:147362-39-8
- Antibiotic PF 1052
Catalog No.:BCN1828
CAS No.:147317-15-5
- KRCA 0008
Catalog No.:BCC8007
CAS No.:1472795-20-2
- 7ACC2
Catalog No.:BCC5554
CAS No.:1472624-85-3
- glatiramer acetate
Catalog No.:BCC5642
CAS No.:147245-92-9
- Delavirdine mesylate
Catalog No.:BCC4069
CAS No.:147221-93-0
- Arecaidine propargyl ester tosylate
Catalog No.:BCC6628
CAS No.:147202-94-6
- N-6-Methyl-7,7-dioxo-2-sulfamoyl-5,6-dihydro-4H-thieno[2,3-b]thiopyran-4-yl]acetamide
Catalog No.:BCC9077
CAS No.:147200-03-1
- Telenzepine dihydrochloride
Catalog No.:BCC6946
CAS No.:147416-96-4
- Ginsenoside Rg6
Catalog No.:BCN2706
CAS No.:147419-93-0
- Pitavastatin
Catalog No.:BCC4140
CAS No.:147511-69-1
- Thunberginol C
Catalog No.:BCN1654
CAS No.:147517-06-4
- LY 288513
Catalog No.:BCC5772
CAS No.:147523-65-7
- Pitavastatin Calcium
Catalog No.:BCC3842
CAS No.:147526-32-7
- Bosentan
Catalog No.:BCC4640
CAS No.:147536-97-8
- trans-3-Hydroxycinnamic acid
Catalog No.:BCN5029
CAS No.:14755-02-3
- Racemodine
Catalog No.:BCN2023
CAS No.:147554-28-7
- DiMNF
Catalog No.:BCC3900
CAS No.:14756-24-2
- ID-8
Catalog No.:BCC4787
CAS No.:147591-46-6
- Novobiocin Sodium
Catalog No.:BCC4812
CAS No.:1476-53-5
Safety, pharmacokinetics, and pharmacodynamics of oral omaveloxolone (RTA 408), a synthetic triterpenoid, in a first-in-human trial of patients with advanced solid tumors.[Pubmed:28919776]
Onco Targets Ther. 2017 Aug 29;10:4239-4250.
BACKGROUND: Omaveloxolone is a semisynthetic oleanane triterpenoid that potently activates Nrf2 with subsequent antioxidant function. We conducted a first-in-human Phase I clinical trial (NCT02029729) with the primary objectives to determine the appropriate dose for Phase II studies, characterize pharmacokinetic and pharmacodynamic parameters, and assess antitumor activity. METHODS: Omaveloxolone was administered orally once daily continuously in a 28-day cycle for patients with stage 4 relapsed/refractory melanoma or non-small cell lung cancer. An accelerated titration design was employed until a grade 2-related adverse event (AE) occurred. A standard 3+3 dose escalation was employed. Single-dose and steady-state plasma pharmacokinetics of the drug were characterized. Downstream Nrf2 activation was assessed in peripheral blood mononuclear cells by quantification of target gene mRNA expression. RESULTS: Omaveloxolone was tested at four dose levels up to 15 mg given orally once daily. No dose-limiting toxicities were detected, and the maximum tolerated dose was not determined. All drug-related AEs were either grade 1 or 2 in severity, and none required clinical action. The most common drug-related AEs were elevated alkaline phosphatase (18%) and anemia (18%). No drug interruptions or reductions were required. Omaveloxolone was rapidly absorbed and exhibited proportional increases in exposure across dose levels. With some exceptions, an overall trend toward time-dependent and dose-dependent activation of Nrf2 antioxidant genes was observed. No confirmed radiologic responses were seen, although one lung cancer subject did have stable disease exceeding 1 year. CONCLUSIONS: Omaveloxolone has favorable tolerability at biologically active doses, although this trial had a small sample size which limits definitive conclusions. These findings support further investigation of omaveloxolone in cancer.


