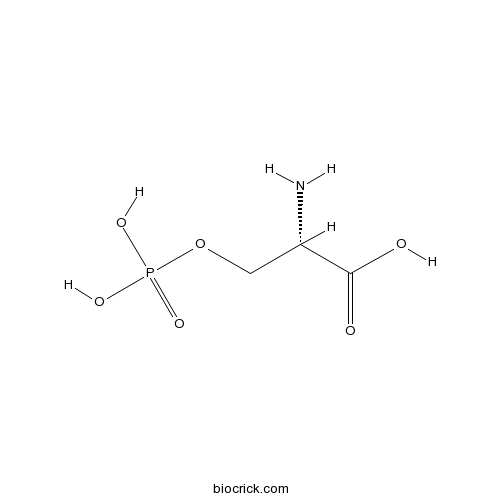
O-Phospho-L-serineGroup III mGlu agonist; enhances differentiation of neural progenitor cells CAS# 407-41-0 |
- Cefditoren Pivoxil
Catalog No.:BCC4898
CAS No.:117467-28-4
- Cefoselis
Catalog No.:BCC4092
CAS No.:122841-10-5
- Balofloxacin
Catalog No.:BCC4892
CAS No.:127294-70-6
- Pefloxacin Mesylate Dihydrate
Catalog No.:BCC5089
CAS No.:149676-40-4
- Tinidazole
Catalog No.:BCC4866
CAS No.:19387-91-8
- Toltrazuril
Catalog No.:BCC4870
CAS No.:69004-03-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 407-41-0 | SDF | Download SDF |
| PubChem ID | 68841 | Appearance | Powder |
| Formula | C3H8NO6P | M.Wt | 185.07 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | L-SOP | ||
| Solubility | H2O : ≥ 16.66 mg/mL (90.02 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (2S)-2-amino-3-phosphonooxypropanoic acid | ||
| SMILES | C(C(C(=O)O)N)OP(=O)(O)O | ||
| Standard InChIKey | BZQFBWGGLXLEPQ-REOHCLBHSA-N | ||
| Standard InChI | InChI=1S/C3H8NO6P/c4-2(3(5)6)1-10-11(7,8)9/h2H,1,4H2,(H,5,6)(H2,7,8,9)/t2-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Group III metabotropic glutamate receptor agonist; analog of L-AP4. Neuroprotective against excitotoxicity in cortical cell cultures. Inhibits proliferation and enhances neuronal differentiation in progenitor cells. |

O-Phospho-L-serine Dilution Calculator

O-Phospho-L-serine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.4034 mL | 27.0168 mL | 54.0336 mL | 108.0672 mL | 135.084 mL |
| 5 mM | 1.0807 mL | 5.4034 mL | 10.8067 mL | 21.6134 mL | 27.0168 mL |
| 10 mM | 0.5403 mL | 2.7017 mL | 5.4034 mL | 10.8067 mL | 13.5084 mL |
| 50 mM | 0.1081 mL | 0.5403 mL | 1.0807 mL | 2.1613 mL | 2.7017 mL |
| 100 mM | 0.054 mL | 0.2702 mL | 0.5403 mL | 1.0807 mL | 1.3508 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
O-Phospho-L-serine is the immediate precursor to L-serine in the serine synthesis pathway, and an agonist at the group III mGluR receptors (mGluR4, mGluR6, mGluR7, and mGluR8); O-Phospho-L-serine also acts as a weak antagonist for mGluR1 and a potent antagonist for mGluR2.
In Vitro:O-Phospho-L-serine (l-SOP) weakly binds to mGluR1, and antagonizes the effects of l-glutamate. l-SOP activates the group III receptors (mGluR4, mGluR6, mGluR7, and mGluR8), but mGluR7 has much lower affinity for l-SOP than the other group III receptors and also displays lower efficacy for both ligands[1]. O-Phospho-L-serine (l-SOP) generates enhanced intracellular calcium responses in mGluR4 transfected cells. l-SOP inhibits the l-glutamate mediated mGluR1 response, with a Ki of 1 mM; l-SOP displays a substantially more potent inhibition of mGluR2 activation, with a Ki of 1 μM, three orders-of-magnitude more potent than for mGluR1. l-SOP induces membrane potential changes in HEK/TRPC4 cells transfected with mGluR4 or mGluR6. l-SOP induces TRPC4β activation mediated by Gαi/o proteins[2]. O-Phospho-L-serine (L-SOP) inhibits Müller glia proliferation, without affecting light-induced photoreceptor cell death. L-SOP disrupts Müller glia proliferation subsequent to or in parallel with the activation of ascl1a and stat3 expression in the light-damaged retina. L-SOP inhibits cone cell regeneration in the light-damaged retina[3].
References:
[1]. Kang HJ, et al. Determinants of endogenous ligand specificity divergence among metabotropic glutamate receptors. J Biol Chem. 2015 Jan 30;290(5):2870-8.
[2]. Kang HJ, et al. Selectivity and evolutionary divergence of metabotropic glutamate receptors for endogenous ligands and G proteins coupled to phospholipase C or TRP channels. J Biol Chem. 2014 Oct 24;289(43):29961-74.
[3]. Bailey TJ, et al. The inhibitor of phagocytosis, O-phospho-L-serine, suppresses Müller glia proliferation and cone cell regeneration in the light-damaged zebrafish retina. Exp Eye Res. 2010 Nov;91(5):601-12.
- Taxifolin 3-O-beta-D-xylopyranoside
Catalog No.:BCN5458
CAS No.:40672-47-7
- Cornoside
Catalog No.:BCN7575
CAS No.:40661-45-8
- IKK-2 inhibitor VIII
Catalog No.:BCC1642
CAS No.:406209-26-5
- ACHP
Catalog No.:BCC6223
CAS No.:406208-42-2
- DSP-4
Catalog No.:BCC7527
CAS No.:40616-75-9
- DMT-T
Catalog No.:BCC2843
CAS No.:40615-39-2
- DMT-Cl
Catalog No.:BCC2799
CAS No.:40615-36-9
- Cirazoline hydrochloride
Catalog No.:BCC6833
CAS No.:40600-13-3
- C34
Catalog No.:BCC5603
CAS No.:40592-88-9
- GW3965 HCl
Catalog No.:BCC3790
CAS No.:405911-17-3
- GW3965
Catalog No.:BCC1612
CAS No.:405911-09-3
- SB590885
Catalog No.:BCC4392
CAS No.:405554-55-4
- Actinine
Catalog No.:BCN1744
CAS No.:407-64-7
- (-)-Bicuculline methiodide
Catalog No.:BCC7387
CAS No.:40709-69-1
- (+)-Nerolidol
Catalog No.:BCC8219
CAS No.:142-50-7
- CaCCinh-A01
Catalog No.:BCC6314
CAS No.:407587-33-1
- 1,7-Diepi-8,15-cedranediol
Catalog No.:BCN5460
CAS No.:40768-81-8
- FPL 55712
Catalog No.:BCC7310
CAS No.:40785-97-5
- PD 146176
Catalog No.:BCC7504
CAS No.:4079-26-9
- H-D-Asp(OBzl)-OBzl.TosOH
Catalog No.:BCC2900
CAS No.:4079-64-5
- MDL 72222
Catalog No.:BCC6733
CAS No.:40796-97-2
- Suprofen
Catalog No.:BCC4943
CAS No.:40828-46-4
- 3,5-Dihydroxy-1-(3,4-dihydroxyphenyl)-7-(4-hydroxyphenyl)heptane
Catalog No.:BCN7165
CAS No.:408324-00-5
- 3,5-Dihydroxy-1,7-bis(3,4-dihydroxyphenyl)heptane
Catalog No.:BCN7494
CAS No.:408324-01-6
Intravenous administration of Factor VIII-O-Phospho-L-Serine (OPLS) complex reduces immunogenicity and preserves pharmacokinetics of the therapeutic protein.[Pubmed:25459532]
Eur J Pharm Sci. 2015 Jan 23;66:157-62.
Hemophilia A is a bleeding disorder caused by the deficiency of an important coagulation factor; Factor VIII (FVIII). Replacement therapy using exogenously administered recombinant FVIII is the most commonly used method of treatment. However, approximately 30% of Hemophilia A patients develop neutralizing antibodies (Nabs) against the recombinant protein. Nabs abolish FVIII activity and drastically influence efficacy of the protein. The immunogenic epitopes of FVIII reside predominantly in the C2 domain of FVIII. However, the C2 domain also contains a lipid binding region. O-Phospho-L-serine (OPLS) which is the head-group moiety of phosphatidylserine, interacts with the lipid binding region of FVIII. Previous studies have shown that FVIII complexed with OPLS lowered Nab development against FVIII following subcutaneous administration. In dendritic cell-T-cell co-culture studies, OPLS treatment increased the secretion of immunosuppressive cytokines (Transforming Growth Factor-beta and Interleukin-10), and simultaneously decreased pro-inflammatory IL-17 cytokine. Here, we investigated FVIII immune response and pharmacokinetics upon intravenous administration of FVIII-OPLS complex. We studied the effect of FVIII-OPLS complex on the interaction between a professional antigen presenting cell; dendritic cell and T-cell, and T-cell clonal expansion. Pharmacokinetics parameters were estimated following intravenous administration of FVIII and FVIII-OPLS. The results suggest that OPLS lowers FVIII immune response following intravenous administration. OPLS also hinders FVIII-specific T-cell clonal proliferation and preserves FVIII PK profile. Thus, the ease of protein-lipid complexation, preservation of FVIII activity and in vivo behavior, and improved in vitro FVIII stability, makes OPLS an attractive excipient in the preparation of next generation or biosimilar FVIII products with improved safety profile.
TrpB2 enzymes are O-phospho-l-serine dependent tryptophan synthases.[Pubmed:25184516]
Biochemistry. 2014 Sep 30;53(38):6078-83.
The rapid increase of the number of sequenced genomes asks for the functional annotation of the encoded enzymes. We used a combined computational-structural approach to determine the function of the TrpB2 subgroup of the tryptophan synthase beta chain/beta chain-like TrpB1-TrpB2 family (IPR023026). The results showed that TrpB2 enzymes are O-Phospho-L-serine dependent tryptophan synthases, whereas TrpB1 enzymes catalyze the l-serine dependent synthesis of tryptophan. We found a single residue being responsible for the different substrate specificities of TrpB1 and TrpB2 and confirmed this finding by mutagenesis studies and crystallographic analysis of a TrpB2 enzyme with bound O-Phospho-L-serine.
Thermostability and reactivity in organic solvent of O-phospho-L-serine sulfhydrylase from hyperthermophilic archaeon Aeropyrum pernix K1.[Pubmed:25779754]
Biosci Biotechnol Biochem. 2015;79(8):1280-6.
O-Phospho-L-serine sulfhydrylase (OPSS) from archaeon Aeropyrum pernix K1 is able to synthesize l-cysteine even at 80 degrees C. In this article, we compared thermal stability and reactivity in organic solvent of OPSS with those of O-acetyl-l-serine sulfhydrylase B (OASS-B) from Escherichia coli. As a result, the thermostability of OPSS was much higher than that of OASS-B. Moreover, the activity of OPSS increased in the reaction mixture containing the organic solvent, such as N, N'-dimethyl formamide and 1,4-dioxane, whereas that of OASS-B gradually decreased as the content of organic solvent increased. From the crystal structural analysis, the intramolecular electrostatic interactions of N-terminal domain in OPSS seemed to be correlated with the tolerance of OPSS to high temperature and organic solvent. These results indicate that OPSS is more superior to OASS-B for the industrial production of l-cysteine and unnatural amino acids that are useful pharmaceuticals in the presence of organic solvent.
O-phospho-l-serine mediates hyporesponsiveness toward FVIII in hemophilia A-murine model by inducing tolerogenic properties in dendritic cells.[Pubmed:25266204]
J Pharm Sci. 2014 Nov;103(11):3457-3463.
The clinical use of therapeutic proteins can be complicated by the development of anti-product antibodies. We have previously observed that O-Phospho-L-serine (OPLS) reduced antibody response to FVIII in Hemophilia-A (HA) mice. However, the mechanism underlying this observation is not clear. We hypothesize that OPLS reduces immunogenicity by inducing tolerogenic properties in dendritic cells (DCs). We tested this hypothesis using in vivo, in vitro, and ex vivo methods. Naive HA mice that were pre-exposed to FVIII in the presence of OPLS showed substantially lower antibody response following rechallenge with OPLS free FVIII as compared with dexamethasone-pretreated mice. Exposure of OPLS to bone-marrow-derived dendritic cells (BMDCs) in culturing conditions resulted in an increase in the regulatory cytokine TGF-beta and a decrease in proinflammatory cytokines TNF-alpha and IL12p70. This was accompanied by a significant reduction in upregulation of costimulatory marker CD40, as measured by flow cytometry. Furthermore, ex vivo matured BMDCs in the presence of FVIII and OPLS failed to elicit a robust immune response in HA mice compared with FVIII-treated BMDCs. Our data suggest that OPLS modulates the immune response by altering the function and maturation of DCs, resulting in the induction of tolerogenic properties. (c) 2014 Wiley Periodicals, Inc. and the American Pharmacists Association J Pharm Sci 103:3457-3463, 2014.
A chemical biology route to site-specific authentic protein modifications.[Pubmed:27708052]
Science. 2016 Nov 4;354(6312):623-626.
Many essential biological processes are controlled by posttranslational protein modifications. The inability to synthetically attain the diversity enabled by these modifications limits functional studies of many proteins. We designed a three-step approach for installing authentic posttranslational modifications in recombinant proteins. We first use the established O-phosphoserine (Sep) orthogonal translation system to create a Sep-containing recombinant protein. The Sep residue is then dephosphorylated to dehydroalanine (Dha). Last, conjugate addition of alkyl iodides to Dha, promoted by zinc and copper, enables chemoselective carbon-carbon bond formation. To validate our approach, we produced histone H3, ubiquitin, and green fluorescent protein variants with site-specific modifications, including different methylations of H3K79. The methylated histones stimulate transcription through histone acetylation. This approach offers a powerful tool to engineer diverse designer proteins.
Expanding the genetic code of Escherichia coli with phosphoserine.[Pubmed:21868676]
Science. 2011 Aug 26;333(6046):1151-4.
O-Phosphoserine (Sep), the most abundant phosphoamino acid in the eukaryotic phosphoproteome, is not encoded in the genetic code, but synthesized posttranslationally. Here, we present an engineered system for specific cotranslational Sep incorporation (directed by UAG) into any desired position in a protein by an Escherichia coli strain that harbors a Sep-accepting transfer RNA (tRNA(Sep)), its cognate Sep-tRNA synthetase (SepRS), and an engineered EF-Tu (EF-Sep). Expanding the genetic code rested on reengineering EF-Tu to relax its quality-control function and permit Sep-tRNA(Sep) binding. To test our system, we synthesized the activated form of human mitogen-activated ERK activating kinase 1 (MEK1) with either one or two Sep residues cotranslationally inserted in their canonical positions (Sep(218), Sep(222)). This system has general utility in protein engineering, molecular biology, and disease research.
A phenotypic small-molecule screen identifies an orphan ligand-receptor pair that regulates neural stem cell differentiation.[Pubmed:17884634]
Chem Biol. 2007 Sep;14(9):1019-30.
High-throughput identification of small molecules that selectively modulate molecular, cellular, or systems-level properties of the mammalian brain is a significant challenge. Here we report the chemical genetic identification of the orphan ligand phosphoserine (P-Ser) as an enhancer of neurogenesis. P-Ser inhibits neural stem cell/progenitor proliferation and self-renewal, enhances neurogenic fate commitment, and improves neuronal survival. We further demonstrate that the effects of P-Ser are mediated by the group III metabotropic glutamate receptor 4 (mGluR4). siRNA-mediated knockdown of mGluR4 abolished the effects of P-Ser and increased neurosphere proliferation, at least in part through upregulation of mTOR pathway activity. We also found that P-Ser increases neurogenesis in human embryonic stem cell-derived neural progenitors. This work highlights the tremendous potential of developing effective small-molecule drugs for use in regenerative medicine or transplantation therapy.
Activation of group III metabotropic glutamate receptors is neuroprotective in cortical cultures.[Pubmed:8880068]
Eur J Pharmacol. 1996 Aug 22;310(1):61-6.
(RS)-alpha-Methyl-4-phosphonophenylglycine (MPPG) and (S)-alpha-methyl-3-carboxyphenylalanine (M3CPA), two novel preferential antagonists of group III metabotropic glutamate (mGlu) receptors, antagonized the neuroprotective activity of L-2-amino-4-phosphono-butanoate (L-AP4) or L-serine-O-phosphate in mice cultured cortical cells exposed to a toxic pulse of N-methyl-D-aspartate. In contrast, MPPG did not influence the neuroprotective activity of the selective group II mGlu receptor agonist, (2S,1'R,2'R,3'R)-2-(2,3-dicarboxy-cyclopropyl) glycine (DCG-IV). These results indicate that activation of group III mGu receptors exerts neuroprotective activity against excitotoxic neuronal death. At least one of the two major group III mGlu receptor subtypes, i.e. mGlu4 receptor, is expressed by cultured cortical neurons, as shown by immunocytochemical analysis with specific polyclonal antibodies.
[3H]-L-2-amino-4-phosphonobutyrate labels a metabotropic glutamate receptor, mGluR4a.[Pubmed:8719808]
Br J Pharmacol. 1995 Dec;116(8):3279-87.
1. The ligand binding site of subtype mGluR4a of the metabotropic glutamate receptor family was characterized by using [3H]-L-2-amino-4-phosphonobutyrate ([3H]-L-AP4) binding. 2. Specific [3H]-L-AP4 binding to membranes prepared from baby hamster kidney (BHK) cells transfected with a vector encoding mGluR4a accounted for 60-70% of the total binding whereas no specific binding of [3H]-L-AP4 was observed to membranes prepared from BHK cells expressing the vector only. 3. Specific binding of [3H]-L-AP4 to mGluR4a was detectable at 0 degree C, was saturated with 10 min and enhanced by Cl(-)-ions but not by divalent cations (Mg2+, Ca2+, Mn2+). 4. [3H]-L-AP4 binding showed a maximal binding density (Bmax) of 3.0 +/- 0.5 pmol mg-1 protein and an affinity (KD) of 441 nM. A modest decrease in affinity was observed in the presence of 0.1 mM guanosine-5'-O-(3-thio)trisphosphate-gamma-S, the KD being 761 nM and the Bmax 3.4 +/- 0.6 pmol mg-1 protein. 5. The following rank order of affinity for mGluR4a was observed: L-AP4 = L-serine-O-phosphate > glutamate = (2S,1S,2S)-2-(carboxycyclopropyl)-glycine > 1-amino-3-(phosphonomethylene)cyclobitanecar-boxylate > > (1S,3R)-1-aminocyclopentane-1,3-dicarboxylate = quisqualate > ibotenate. 6. A highly significant correlation was observed between the potencies of the compounds to inhibit forskolin-stimulated cyclic AMP-formation in BHK cells expressing mGluR4a and the affinity for displacement of [3H]-L-AP4 binding from mGluR4a suggesting that this binding site is functionally relevant. 7. In conclusion, [3H]-L-AP4 is a suitable radioligand for characterizing mGluR4a when expressed in BHK cells. Interestingly, a significant correlation was found between the ability of various compounds to displace [3H]-L-AP4 binding from mGluR4a and the previously observed potencies for inhibition of synaptic transmission via L-AP4 sensitive glutamatergic pathways. These data support the hypothesis that the L-AP4 receptor is contained within the mGluR family.
Pharmacology of selective and non-selective metabotropic glutamate receptor agonists at L-AP4 receptors in retinal ON bipolar cells.[Pubmed:7796182]
Brain Res. 1995 Apr 3;676(1):93-102.
Retinal ON bipolar cells possess metabotropic glutamate receptors (mGluRs) which are sensitive to L-2-amino-4-phosphonobutyric acid (L-AP4). Recent studies suggest there are multiple subtypes of L-AP4 receptors. In order to provide a more complete description of the pharmacology of the retinal L-AP4 receptor, we examined the actions of a number of compounds which are active at L-AP4 receptors and other mGluRs. Four groups of compounds were studied: (1) AP4 analogues (e.g. L-AP5, L-SOP, cyclobutylene AP5, and N-Me-AP4), (2) non-selective mGluR agonists (ibotenate and quisqualate), (3) selective mGluR agonists (L-CCG-I), and (4) agonists proposed to be selective for specific mGluR subtypes (DCG-IV and t-ADA). Concentration-response curves were obtained using the b-wave of the electroretinogram (ERG) as an assay for L-AP4 receptor activation. Whole cell voltage clamp recordings from ON bipolar cells in the retinal slice preparation of the mudpuppy were used to determine whether the compounds acted as L-AP4 receptor agonists. All compounds were L-AP4 receptor agonists, except t-ADA which was ineffective. The results reveal pharmacological differences between L-AP4 receptors in mudpuppy ON bipolar cells and those in other systems, consistent with the proposal that there are multiple L-AP4 receptor subtypes. For example, retinal L-AP4 receptors are more potently activated by L-AP5 than L-SOP, whereas L-SOP has been shown to be more potent than L-AP5 in L-AP4 receptors in the lateral perforant path (LPP) of the rat hippocampus. L-SOP is also relatively more potent at the cloned L-AP4 receptors mGluR4, 6, and 7 than in mudpuppy ON bipolar cells in situ. The different potencies of these compounds in retina and LPP is ascribed to both steric and charge factors. The results with DCG-IV and t-ADA are consistent with the proposal that these are subtype-selective agonists, but DCG-IV is likely to be selective only at very low concentrations (< or = 1 microM).


