DMT-ClCAS# 40615-36-9 |
- ML347
Catalog No.:BCC5331
CAS No.:1062368-49-3
- LDN-212854
Catalog No.:BCC5330
CAS No.:1432597-26-6
- PD 169316
Catalog No.:BCC3969
CAS No.:152121-53-4
- Imperatorin
Catalog No.:BCN5574
CAS No.:482-44-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

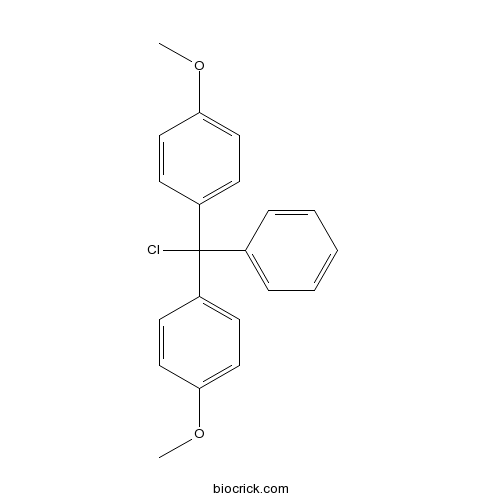
| Cas No. | 40615-36-9 | SDF | Download SDF |
| PubChem ID | 96831 | Appearance | Powder |
| Formula | C21H19ClO2 | M.Wt | 338.8 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in water or 1% acetic acid | ||
| Chemical Name | 1-[chloro-(4-methoxyphenyl)-phenylmethyl]-4-methoxybenzene | ||
| SMILES | COC1=CC=C(C=C1)C(C2=CC=CC=C2)(C3=CC=C(C=C3)OC)Cl | ||
| Standard InChIKey | JBWYRBLDOOOJEU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H19ClO2/c1-23-19-12-8-17(9-13-19)21(22,16-6-4-3-5-7-16)18-10-14-20(24-2)15-11-18/h3-15H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

DMT-Cl Dilution Calculator

DMT-Cl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9516 mL | 14.758 mL | 29.5159 mL | 59.0319 mL | 73.7898 mL |
| 5 mM | 0.5903 mL | 2.9516 mL | 5.9032 mL | 11.8064 mL | 14.758 mL |
| 10 mM | 0.2952 mL | 1.4758 mL | 2.9516 mL | 5.9032 mL | 7.379 mL |
| 50 mM | 0.059 mL | 0.2952 mL | 0.5903 mL | 1.1806 mL | 1.4758 mL |
| 100 mM | 0.0295 mL | 0.1476 mL | 0.2952 mL | 0.5903 mL | 0.7379 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
DMT-Cl
- Cirazoline hydrochloride
Catalog No.:BCC6833
CAS No.:40600-13-3
- C34
Catalog No.:BCC5603
CAS No.:40592-88-9
- GW3965 HCl
Catalog No.:BCC3790
CAS No.:405911-17-3
- GW3965
Catalog No.:BCC1612
CAS No.:405911-09-3
- SB590885
Catalog No.:BCC4392
CAS No.:405554-55-4
- Cyclapolin 9
Catalog No.:BCC7571
CAS No.:40533-25-3
- Dadahol A
Catalog No.:BCN5457
CAS No.:405281-76-7
- NFPS
Catalog No.:BCC7484
CAS No.:405225-21-0
- Dovitinib (TKI-258, CHIR-258)
Catalog No.:BCC1169
CAS No.:405169-16-6
- CHIR-124
Catalog No.:BCC3750
CAS No.:405168-58-3
- Besifloxacin HCl
Catalog No.:BCC4764
CAS No.:405165-61-9
- Drechslerine D
Catalog No.:BCN7502
CAS No.:405157-88-2
- DMT-T
Catalog No.:BCC2843
CAS No.:40615-39-2
- DSP-4
Catalog No.:BCC7527
CAS No.:40616-75-9
- ACHP
Catalog No.:BCC6223
CAS No.:406208-42-2
- IKK-2 inhibitor VIII
Catalog No.:BCC1642
CAS No.:406209-26-5
- Cornoside
Catalog No.:BCN7575
CAS No.:40661-45-8
- Taxifolin 3-O-beta-D-xylopyranoside
Catalog No.:BCN5458
CAS No.:40672-47-7
- O-Phospho-L-serine
Catalog No.:BCC6578
CAS No.:407-41-0
- Actinine
Catalog No.:BCN1744
CAS No.:407-64-7
- (-)-Bicuculline methiodide
Catalog No.:BCC7387
CAS No.:40709-69-1
- (+)-Nerolidol
Catalog No.:BCC8219
CAS No.:142-50-7
- CaCCinh-A01
Catalog No.:BCC6314
CAS No.:407587-33-1
- 1,7-Diepi-8,15-cedranediol
Catalog No.:BCN5460
CAS No.:40768-81-8
Unusual C7- versus Normal 5'-O-Dimethoxytritylation of 6-Arylpyrrolocytidine Analogs.[Pubmed:27529362]
J Org Chem. 2016 Sep 16;81(18):8415-25.
Fluorescent deoxynucleosides possessing the modified bases 6-(2-benzo[b]furyl)- and 6-(2-furyl)pyrrolocytosine (BFpC and FpC) have been synthesized along with the quencher nucleosides possessing 6-{4-[(4-dimethylamino)azo]phenyl}pyrrolocytosine (DABCYLpC) and 6-(p-nitrophenyl)pyrrolocytosine (p-NO2-PhpC) nucleobase analogs. Standard treatment of BFpC, FpC, DABCYLpC, and p-NO2-PhpC with dimethoxytrityl chloride (DMT-Cl) led to the unusual substitution on the C7 of the pyrrolocytosine skeleton. The desired 5'-O-DMT-protected nucleoside analogs were synthesized from suitably protected 5'-O-DMT cytidines. Subsequent phosphitylation smoothly afforded BFpC-, FpC-, DABCYLpC-, and p-NO2-PhpC-derived monomers suitable for standard oligonucleotide synthesis.


