Tropic acidCAS# 529-64-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 529-64-6 | SDF | Download SDF |
| PubChem ID | 10726 | Appearance | Powder |
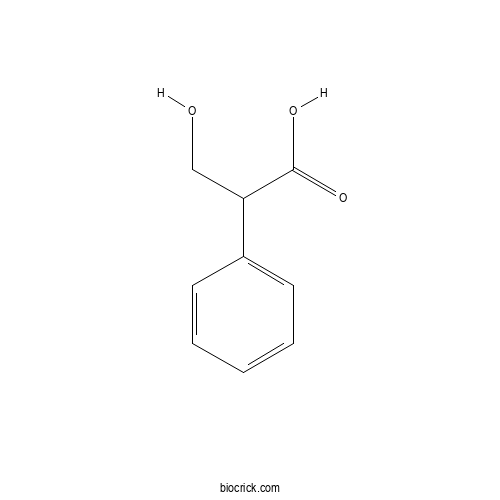
| Formula | C9H10O3 | M.Wt | 166.2 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-hydroxy-2-phenylpropanoic acid | ||
| SMILES | C1=CC=C(C=C1)C(CO)C(=O)O | ||
| Standard InChIKey | JACRWUWPXAESPB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H10O3/c10-6-8(9(11)12)7-4-2-1-3-5-7/h1-5,8,10H,6H2,(H,11,12) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Reference standards. | |||||

Tropic acid Dilution Calculator

Tropic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.0168 mL | 30.0842 mL | 60.1685 mL | 120.3369 mL | 150.4212 mL |
| 5 mM | 1.2034 mL | 6.0168 mL | 12.0337 mL | 24.0674 mL | 30.0842 mL |
| 10 mM | 0.6017 mL | 3.0084 mL | 6.0168 mL | 12.0337 mL | 15.0421 mL |
| 50 mM | 0.1203 mL | 0.6017 mL | 1.2034 mL | 2.4067 mL | 3.0084 mL |
| 100 mM | 0.0602 mL | 0.3008 mL | 0.6017 mL | 1.2034 mL | 1.5042 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-(4-Methoxyphenyl)-2-butanone
Catalog No.:BCN9865
CAS No.:104-20-1
- ICG-001
Catalog No.:BCN9864
CAS No.:780757-88-2
- 3,29-O-Dibenzoyloxykarounidiol
Catalog No.:BCN9863
CAS No.:389122-01-4
- Somnifericin
Catalog No.:BCN9862
CAS No.:173693-57-7
- Solanidine
Catalog No.:BCN9861
CAS No.:80-78-4
- Sphenanlignan
Catalog No.:BCN9860
CAS No.:866347-36-6
- Vanillic acid glucoside
Catalog No.:BCN9859
CAS No.:32142-31-7
- alpha-Peltatin
Catalog No.:BCN9858
CAS No.:568-53-6
- 5,6-Benzoflavone
Catalog No.:BCN9857
CAS No.:6051-87-2
- Quasipanaxatriol
Catalog No.:BCN9856
CAS No.:171903-78-9
- n-Tridecane
Catalog No.:BCN9855
CAS No.:629-50-5
- 3-Hydroxybenzoic acid
Catalog No.:BCN9854
CAS No.:99-06-9
- 1,2-Dimethoxybenzene
Catalog No.:BCN9867
CAS No.:91-16-7
- 15-Methoxy-16-oxo-15,16H-strictic acid
Catalog No.:BCN9868
CAS No.:1356388-38-9
- Datiscin
Catalog No.:BCN9869
CAS No.:16310-92-2
- Cannabiscitrin
Catalog No.:BCN9870
CAS No.:520-14-9
- Lappaconine
Catalog No.:BCN9871
CAS No.:23943-93-3
- Condurango glycoside A
Catalog No.:BCN9872
CAS No.:11051-90-4
- 2-(4-Hydroxybenzal)acetophenone
Catalog No.:BCN9873
CAS No.:20426-12-4
- Norharman
Catalog No.:BCN9874
CAS No.:244-63-3
- Syringetin
Catalog No.:BCN9875
CAS No.:4423-37-4
- 4-Hydroxyisophthalic acid
Catalog No.:BCN9876
CAS No.:636-46-4
- Lyciumamide B
Catalog No.:BCN9877
CAS No.:1647111-41-8
- Tricaprin
Catalog No.:BCN9878
CAS No.:621-71-6
Stability of Ophthalmic Atropine Solutions for Child Myopia Control.[Pubmed:32824572]
Pharmaceutics. 2020 Aug 17;12(8). pii: pharmaceutics12080781.
Myopia is an ophthalmic condition affecting more than 1/5th of the world population, especially children. Low-dose atropine eyedrops have been shown to limit myopia evolution during treatment. However, there are currently no commercial industrial forms available and there is little data published concerning the stability of medications prepared by compounding pharmacies. The objective of this study was to evaluate the stability of two 0.1 mg/mL atropine formulations (with and without antimicrobiobial preservatives) for 6 months in two different low-density polyethylene (LDPE) multidose eyedroppers. Analyses used were the following: visual inspection, turbidity, chromaticity measurements, osmolality and pH measurements, atropine quantification by a stability-indicating liquid chromatography method, breakdown product research, and sterility assay. In an in-use study, atropine quantification was also performed on the drops emitted from the multidose eyedroppers. All tested parameters remained stable during the 6 months period, with atropine concentrations above 94.7% of initial concentration. A breakdown product (Tropic acid) did increase slowly over time but remained well below usually admitted concentrations. Atropine concentrations remained stable during the in-use study. Both formulations of 0.1 mg/mL of atropine (with and without antimicrobial preservative) were proved to be physicochemically stable for 6 months at 25 degrees C when stored in LDPE bottles, with an identical microbial shelf-life.
Buscopan labeled with carbon-14 and deuterium.[Pubmed:27753138]
J Labelled Comp Radiopharm. 2016 Nov;59(13):557-564.
Hyosine butyl bromide, the active ingredient in Buscopan, is an anticholinergic and antimuscarinic drug used to treat pain and discomfort caused by abdominal cramps. A straightforward synthesis of carbon-14- and deuterium-labeled Buscopan was developed using scopolamine, n-butyl-1-(14) C bromide, and n-butyl-(2) H9 bromide, respectively. In a second carbon-14 synthesis, the radioactive carbon was incorporated in the Tropic acid moiety to follow its metabolism. Herein, we describe the detailed preparations of carbon-14- and deuterium-labeled Buscopan.
Preparative Enantioseparation of beta-Substituted-2-Phenylpropionic Acids by Countercurrent Chromatography With Substituted beta-Cyclodextrin as Chiral Selectors.[Pubmed:26333843]
Chirality. 2015 Nov;27(11):795-801.
Preparative enantioseparation of four beta-substituted-2-phenylpropionic acids was performed by countercurrent chromatography with substituted beta-cyclodextrin as chiral selectors. The two-phase solvent system was composed of n-hexane-ethyl acetate-0.10 mol L-1 of phosphate buffer solution at pH 2.67 containing 0.10 mol L(-1) of hydroxypropyl-beta-cyclodextrin (HP-beta-CD) or sulfobutylether-beta-cyclodextrin (SBE-beta-CD). The influence factors, including the type of substituted beta-cyclodextrin, composition of organic phase, concentration of chiral selector, pH value of the aqueous phase, and equilibrium temperature were optimized by enantioselective liquid-liquid extraction. Under the optimum separation conditions, 100 mg of 2-phenylbutyric acid, 100 mg of Tropic acid, and 50 mg of 2,3-diphenylpropionic acid were successfully enantioseparated by high-speed countercurrent chromatography, and the recovery of the (+/-)-enantiomers was in the range of 90-91% for (+/-)-2-phenylbutyric acid, 91-92% for (+/-)-Tropic acid, 85-87% for (+/-)-2,3-diphenylpropionic acid with purity of over 97%, 96%, and 98%, respectively. The formation of 1:1 stoichiometric inclusion complex of beta-substituted-2-phenylpropionic acids with HP-beta-CD was determined by UV spectrophotometry and the inclusion constants were calculated by a modified Benesi-Hildebrand equation. The results showed that different enantioselectivities among different racemates were mainly caused by different enantiorecognition between each enantiomer and HP-beta-CD, while it might be partially caused by different inclusion capacity between racemic solutes and HP-beta-CD.
The effect of transdermal scopolamine for the prevention of postoperative nausea and vomiting.[Pubmed:24782768]
Front Pharmacol. 2014 Apr 9;5:55.
Postoperative nausea and vomiting (PONV) is one of the most common and undesirable complaints recorded in as many as 70-80% of high-risk surgical patients. The current prophylactic therapy recommendations for PONV management stated in the Society of Ambulatory Anesthesia (SAMBA) guidelines should start with monotherapy and patients at moderate to high risk, a combination of antiemetic medication should be considered. Consequently, if rescue medication is required, the antiemetic drug chosen should be from a different therapeutic class and administration mode than the drug used for prophylaxis. The guidelines restrict the use of dexamethasone, transdermal scopolamine, aprepitant, and palonosetron as rescue medication 6 h after surgery. In an effort to find a safer and reliable therapy for PONV, new drugs with antiemetic properties and minimal side effects are needed, and scopolamine may be considered an effective alternative. Scopolamine is a belladonna alkaloid, alpha-(hydroxymethyl) benzene acetic acid 9-methyl-3-oxa-9-azatricyclo non-7-yl ester, acting as a non-selective muscarinic antagonist and producing both peripheral antimuscarinic and central sedative, antiemetic, and amnestic effects. The empirical formula is C17H21NO4 and its structural formula is a tertiary amine L-(2)-scopolamine (Tropic acid ester with scopine; MW = 303.4). Scopolamine became the first drug commercially available as a transdermal therapeutic system used for extended continuous drug delivery during 72 h. Clinical trials with transdermal scopolamine have consistently demonstrated its safety and efficacy in PONV. Thus, scopolamine is a promising candidate for the management of PONV in adults as a first line monotherapy or in combination with other drugs. In addition, transdermal scopolamine might be helpful in preventing postoperative discharge nausea and vomiting owing to its long-lasting clinical effects.
Crystal structures of CbpF complexed with atropine and ipratropium reveal clues for the design of novel antimicrobials against Streptococcus pneumoniae.[Pubmed:24036328]
Biochim Biophys Acta. 2014 Jan;1840(1):129-35.
BACKGROUND: Streptococcus pneumoniae is a major pathogen responsible of important diseases worldwide such as pneumonia and meningitis. An increasing resistance level hampers the use of currently available antibiotics to treat pneumococcal diseases. Consequently, it is desirable to find new targets for the development of novel antimicrobial drugs to treat pneumococcal infections. Surface choline-binding proteins (CBPs) are essential in bacterial physiology and infectivity. In this sense, esters of bicyclic amines (EBAs) such as atropine and ipratropium have been previously described to act as choline analogs and effectively compete with teichoic acids on binding to CBPs, consequently preventing in vitro pneumococcal growth, altering cell morphology and reducing cell viability. METHODS: With the aim of gaining a deeper insight into the structural determinants of the strong interaction between CBPs and EBAs, the three-dimensional structures of choline-binding protein F (CbpF), one of the most abundant proteins in the pneumococcal cell wall, complexed with atropine and ipratropium, have been obtained. RESULTS: The choline analogs bound both to the carboxy-terminal module, involved in cell wall binding, and, unexpectedly, also to the amino-terminal module, that possesses a regulatory role in pneumococcal autolysis. CONCLUSIONS: Analysis of the complexes confirmed the importance of the Tropic acid moiety of the EBAs on the strength of the binding, through pi-pi interactions with aromatic residues in the binding site. GENERAL SIGNIFICANCE: These results represent the first example describing the molecular basis of the inhibition of CBPs by EBA molecules and pave the way for the development of new generations of antipneumococcal drugs.
Rapid resolution liquid chromatography for monitoring the quality of stockpiled atropine preparations for injection.[Pubmed:22467254]
Drug Test Anal. 2012 Mar-Apr;4(3-4):222-8.
We describe a rapid resolution liquid chromatography (RRLC) method for analyzing atropine sulfate, its degradation products (Tropic acid, apoatropine, aTropic acid) and other components (e.g. phenol, methylparaben) in injectable medicines that are used by the German armed forces in emergency situations. Chromatography is performed using an acetonitrile/phosphate buffer gradient (pH = 1.0) and an RP 18 column (50 x 4.6 mm, 1.8 microm) with the detection wavelength set at 220 nm. The concentration of the active ingredient (atropine sulfate) in the tested products ranges from about 1 mg*ml(-1) to 10 mg*ml(-1) . The concentrations of the detected degradation products range from 0.2% to 4.7% (Tropic acid) in relation to the active pharmaceutical ingredient (API). Using shorter separation columns and smaller particle sizes of the stationary phase improved analysis time from 40 to 10 min and reduced the consumption of solvents by approximately 75%. Owing to the pressure conditions (< 200 bar), UHPLC (ultra high performance liquid chromatography) systems are not needed. Comparison of the atropine and Tropic acid results obtained with the previously used HPLC (high performance liquid chromatography) method of the MAH (marketing authorization holder) show that there is no indication of a significant difference between the two methods.
High-performance liquid-chromatographic tandem-mass spectrometric methods for atropinesterase-mediated enantioselective and chiral determination of R- and S-hyoscyamine in plasma.[Pubmed:20969988]
Anal Chim Acta. 2010 Nov 8;680(1-2):32-40.
S-hyoscyamine (S-hyo) is a toxic tropane alkaloid from plants of the solanacea family, which is extracted for pharmaceutical purposes thereby undergoing racemization (atropine). Merely the S-hyo enantiomer acts as an antagonist of muscarinic receptors (MR). Nevertheless, racemic atropine is clinically administered in e.g. ophthalmology and for symptomatic therapy of acute poisoning with organophosphorus compounds (OPCs, e.g. pesticides, nerve agents). However, very limited data are available of comparative pharmacokinetics of S- and R-enantiomers in humans or other species. Therefore, we developed an enantioselective LC-ESI-MS/MS assay making use of rabbit serum containing atropinesterase (AtrE, EC 3.1.1.10) which is suitable for stereospecific hydrolysis of S-hyo into tropine and Tropic acid while R-hyo is unaffected. For sample preparation plasma was incubated with human serum (not containing AtrE, procedure A) and with rabbit serum (procedure B). Afterwards, hyoscyamines were quantified by a validated previously published non-chiral LC-ESI-MS/MS method. Following procedure A the concentration of total hyo and following procedure B remaining R-hyo were determined. S-hyo was calculated by the difference between these concentrations. This assay design allowed reproducible, precise (RSD 2-9%), accurate (93-101%) and selective determination of total and individual hyoscyamines. Potential therapeutics for OPC poisoning (carbamates, oximes) and thiono-pesticides did not interfere with the assay whereas some oxon-pesticides inhibited S-hyo hydrolysis. A control experiment was designed allowing to be aware of such interferences thus avoiding the use of false results. To validate this assay, results were compared to those from a novel isocratic chiral LC-ESI-MS/MS method. Separation of S-hyo (t(R) 31.1 +/- 0.2 min) and R-hyo (t(R) 33.4 +/- 0.2 min) was achieved on alpha-glycoprotein (AGP) chiral stationary phase at 40 degrees C (selectivity factor alpha 1.07). Ammoniumformate (0.01 M, pH 8.0) with 3.75% (v/v) acetonitrile served as mobile phase (300 muL min(-1)). Hyoscyamines were detected in the positive multiple reaction monitor mode. The enantioselective assay was applied to the analysis of atropine degradation in diluted rabbit serum in vitro as well as to human in vivo plasma samples from a pesticide-poisoned patient treated with atropine.
Determination of hydroxyurea in serum or plasma using gas chromatography-mass spectrometry (GC-MS).[Pubmed:20077079]
Methods Mol Biol. 2010;603:279-87.
Hydroxyurea is an antineoplastic drug, which is also widely used in the treatment of sickle cell disease. Various methods including colorimetry, high performance liquid chromatography, and gas chromatography-mass spectrometry (GC-MS) are available for the assay of hydroxyurea. In the gas chromatography method described, the drug is extracted from serum, plasma, or urine using ethyl acetate and phosphate buffer (pH 6). The organic phase containing drug is separated and dried under a stream of nitrogen. After trimethylsilyl derivatization, samples are analyzed using GC-MS. Quantitation of the drug in a sample is achieved by comparing responses of the unknown sample to the responses of the calibrators using selected ion monitoring. Tropic acid is used as an internal standard.
Occurrence and behavior of system peaks in RP HPLC with solely aqueous mobile phases.[Pubmed:19639550]
J Sep Sci. 2009 Sep;32(17):2864-70.
System peaks are important but often also disturbing phenomena occurring in separation systems. Behavior of system peaks was studied in reversed phase high performance liquid chromatography (RP HPLC) systems consisting of an RP Amide C16 column and aqueous solutions of organic acids with alkaline metal hydroxides as mobile phases. Binary mobile phases, composed of benzoic acid and lithium hydroxide (LiOH) or cesium hydroxide (CsOH), yielded two system peaks. The first peak was stationary and the second one moved with dilution of the mobile phase or with changes of the alkaline metal hydroxide concentration. The latter changes affected dissociation of the benzoic acid present in the mobile phase and thereby its retention. The presumption that the first system peak is not influenced by the type of alkaline metal cation and that it is related to the non-adsorbed component of the mobile phase was confirmed by a cyclic procedure. Three-component mobile phases composed of benzoic acid, Tropic acid, and a hydroxide gave rise to three system peaks as expected. The first peak was again stationary and the two others shifted depending on the concentration variation of both acids. Resonance causing a zigzag peak, well described in capillary zone electrophoresis (CZE), was observed if 1-pentanol was injected into a chromatographic system with one-component mobile phase.
Analysis of scopolamine and its eighteen metabolites in rat urine by liquid chromatography-tandem mass spectrometry.[Pubmed:18970269]
Talanta. 2005 Oct 31;67(5):984-91.
A rapid and sensitive method is described for the determination of scopolamine and its metabolites in rat urine by combining liquid chromatography and tandem mass spectrometry (LC-MS/MS). Various extraction techniques (free fraction, acid hydrolyses and enzyme hydrolyses) and their comparison were carried out for investigation of the metabolism of scopolamine. After extraction procedure, the pretreated samples were injected into a reversed-phase C18 column with mobile phase of methanol/ ammonium acetate (2mM, adjusted to pH 3.5 with formic acid) (70:30, v/v) and detected by an on-line MS/MS system. Identification and structural elucidation of the metabolites were performed by comparing their changes in molecular masses (DeltaM), retention-times and full scan MS(n) spectra with those of the parent drug. The results revealed that at least 18 metabolites (norscopine, scopine, Tropic acid, aponorscopolamine, aposcopolamine, norscopolamine, hydroxyscopolamine, hydroxyscopolamine N-oxide, p-hydroxy-m-methoxyscopolamine, trihydroxyscopolamine, dihydroxy-methoxyscopolamine, hydroxyl-dimethoxyscopolamine, glucuronide conjugates and sulfate conjugates of norscopolamine, hydroxyscopolamine and the parent drug) and the parent drug existed in urine after ingesting 55mg/kg scopolamine to healthy rats. Hydroxyscopolamine, p-hydroxy-m-methoxyscopolamine and the parent drug were detected in rat urine for up 106h after ingestion of scopolamine.
Liquid chromatography-electrospray ionization ion trap mass spectrometry for analysis of in vivo and in vitro metabolites of scopolamine in rats.[Pubmed:18218192]
J Chromatogr Sci. 2008 Jan;46(1):74-80.
In vivo and in vitro metabolism of scopolamine is investigated using a highly specific and sensitive liquid chromatography-mass spectrometry (LC-MSn) method. Feces, urine, and plasma samples are collected individually after ingestion of 55 mg/kg scopolamine by healthy rats. Rat feces and urine samples are cleaned up by a liquid-liquid extraction and a solid-phase extraction procedure (C18 cartridges), respectively. Methanol is added to rat plasma samples to precipitate plasma proteins. Scopolamine is incubated with homogenized liver and intestinal flora of rats in vitro, respectively. The metabolites in the incubating solution are extracted with ethyl acetate. Then these pretreated samples are injected into a reversed-phase C18 column with mobile phase of methanol-ammonium acetate (2 mM, adjusted to pH 3.5 with formic acid) (70:30, v/v) and detected by an on-line MSn system. Identification and structural elucidation of the metabolites are performed by comparing their changes in molecular masses (DeltaM), retention-times and full scan MSn spectra with those of the parent drug. The results reveal that at least 8 metabolites (norscopine, scopine, Tropic acid, aponorscopolamine, aposcopolamine, norscopolamine, hydroxyscopolamine, and hydroxyscopolamine N-oxide) and the parent drug exist in feces after administering 55 mg/kg scopolamine to healthy rats. Three new metabolites (tetrahydroxyscopolamine, trihydroxy-methoxyscopolamine, and dihydroxy-dimethoxyscopolamine) are identified in rat urine. Seven metabolites (norscopine, scopine, Tropic acid, aponorscopolamine, aposcopolamine, norscopolamine, and hydroxyscopolamine) and the parent drug are detected in rat plasma. Only 1 hydrolyzed metabolite (scopine) is found in the rat intestinal flora incubation mixture, and 2 metabolites (aposcopolamine and norscopolamine) are identified in the homogenized liver incubation mixture.


