NelarabineProdrug of ara-G for T-LBL/T-ALL CAS# 121032-29-9 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
- Carboplatin
Catalog No.:BCC1170
CAS No.:41575-94-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 121032-29-9 | SDF | Download SDF |
| PubChem ID | 151121 | Appearance | Powder |
| Formula | C11H15N5O5 | M.Wt | 297.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 506U78; GW 506U78; Nelzarabine | ||
| Solubility | DMSO : 9.8 mg/mL (32.97 mM; Need ultrasonic and warming) | ||
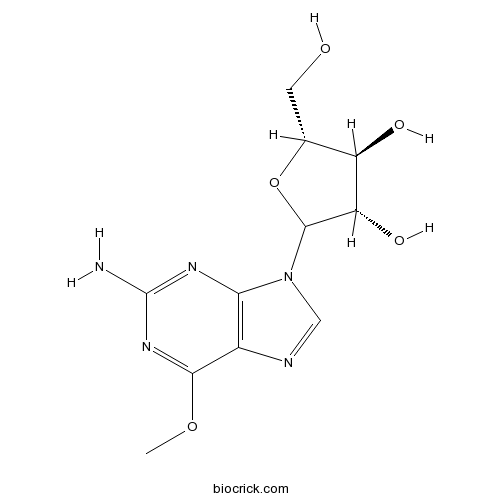
| Chemical Name | (3S,4S,5R)-2-(2-amino-6-methoxypurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol | ||
| SMILES | COC1=NC(=NC2=C1N=CN2C3C(C(C(O3)CO)O)O)N | ||
| Standard InChIKey | IXOXBSCIXZEQEQ-KBNQYOMWSA-N | ||
| Standard InChI | InChI=1S/C11H15N5O5/c1-20-9-5-8(14-11(12)15-9)16(3-13-5)10-7(19)6(18)4(2-17)21-10/h3-4,6-7,10,17-19H,2H2,1H3,(H2,12,14,15)/t4-,6-,7+,10?/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Nucleoside analog. Metabolized by adenosine deaminase to active metabolite, arabinofuranosylguanine (ara-G). Inhibits DNA synthesis and induces apoptosis. Inhibits growth of human T cell lymphoblastic leukemia cells in vitro and in vivo. |

Nelarabine Dilution Calculator

Nelarabine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3639 mL | 16.8197 mL | 33.6395 mL | 67.2789 mL | 84.0986 mL |
| 5 mM | 0.6728 mL | 3.3639 mL | 6.7279 mL | 13.4558 mL | 16.8197 mL |
| 10 mM | 0.3364 mL | 1.682 mL | 3.3639 mL | 6.7279 mL | 8.4099 mL |
| 50 mM | 0.0673 mL | 0.3364 mL | 0.6728 mL | 1.3456 mL | 1.682 mL |
| 100 mM | 0.0336 mL | 0.1682 mL | 0.3364 mL | 0.6728 mL | 0.841 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Nelarabine is a novel and prodrug of ara-G (9-β-d-arabinofuranosylguanine), and it is used to treat T-cell lymphoblastic lymphoma (T-LBL) and T-cell acute lymphoblastic leukemia (T-ALL), which don’t have response after treatment with 2 chemotherapy regimens.[1]
It acts as an inhibitor by inhibiting DNA synthesis and as an inducer by inducing apoptosis in malignant cells. Nelarabine requires demethylation by adenosine deaminase (ADA) in plasma to form the active compound, ara-G. Intracellular by deoxyguanosine kinase and deoxycytidine kinase phosphorylate ara-G to its triphosphate form, ara-GTP. Inside the cell exposure to ara-GTP is much higher than the exposure to ara-G or nelarabine. The substitution of ara-GTP for GTP leads to inhibition of DNA synthesis resulting in cell death by apoptosis. This process is lethal to malignant T cells, as it is to other rapidly replicating cells.[2]
References:
[1] Cohen MH, Johnson JR, Massie T, et al. Approval summary: nelarabine for the treatment of T-cell lymphoblastic leukemia/lymphoma. Clin Cancer Res. September 2006. 18:5329–35.
[2] Yesid Alvarado, Mary Alma Welch, Ronan Swords, John Bruzzi, Ellen Schlette, Francis J. Giles. Nelarabine activity in acute biphenotypic leukemia. Leukemia Research. November 2007. 31(11): 1600-1603.
- Secretin (rat)
Catalog No.:BCC5848
CAS No.:121028-49-7
- JZL 195
Catalog No.:BCC7966
CAS No.:1210004-12-8
- N-Acetyl-5-Hydroxytryptamine
Catalog No.:BCC9080
CAS No.:1210-83-9
- ST 1936 oxalate
Catalog No.:BCC7919
CAS No.:1210-81-7
- 3'-Nitroacetophenone
Catalog No.:BCN2256
CAS No.:121-89-1
- Propyl gallate
Catalog No.:BCN8431
CAS No.:121-79-9
- 2-Amino-5-nitrothiazole
Catalog No.:BCC8538
CAS No.:121-66-4
- N-Acetylsulfanilyl chloride
Catalog No.:BCC9084
CAS No.:121-60-8
- Benzethonium Chloride
Catalog No.:BCC4635
CAS No.:121-54-0
- (-)-Terreic acid
Catalog No.:BCC7051
CAS No.:121-40-4
- Vanillic acid
Catalog No.:BCN6105
CAS No.:121-34-6
- Vanillin
Catalog No.:BCN2605
CAS No.:121-33-5
- IEM 1460
Catalog No.:BCC7135
CAS No.:121034-89-7
- PF-04971729
Catalog No.:BCC1852
CAS No.:1210344-57-2
- Abiesadine I
Catalog No.:BCN6104
CAS No.:1210347-50-4
- Melanotan II
Catalog No.:BCC7414
CAS No.:121062-08-6
- 3-O-trans-p-Coumaroyltormentic acid
Catalog No.:BCN4724
CAS No.:121064-78-6
- 3-O-cis-p-Coumaroyltormentic acid
Catalog No.:BCN3184
CAS No.:121072-40-0
- L-670,596
Catalog No.:BCC5857
CAS No.:121083-05-4
- EG00229
Catalog No.:BCC5376
CAS No.:1210945-69-9
- Cefprozil hydrate
Catalog No.:BCC4951
CAS No.:121123-17-9
- LEE011
Catalog No.:BCC3926
CAS No.:1211441-98-3
- LEE011 hydrochloride
Catalog No.:BCC4101
CAS No.:1211443-80-9
- Rauvoyunine C
Catalog No.:BCN4833
CAS No.:1211543-01-9
Nelarabine-associated reversible Guillain-Barre-like syndrome or myelopathy in an adult patient with primary refractory T-lymphoblastic lymphoma.[Pubmed:28169005]
Curr Probl Cancer. 2017 Mar - Apr;41(2):138-143.
Nelarabine is a purine analogue used for the treatment of patients with relapsed or refractory T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma mainly as a bridge to stem cell transplantation. The water-soluble prodrug of 9-beta-D-arabinofuranosyl guanine (Ara-G) is phosphorylated within leukemic cells to form ara-G triphosphate (ara-GTP), which terminates DNA chain elongation, resulting in cell death. The drug received accelerated approval by the US Food and Drug Administration (FDA) on October 2005 based on the induction of complete remissions in 2 phase II trials. In these trials, neurologic toxicity was mainly presented as peripheral neuropathy and, since, is a commonly reported side effect of the drug. However, cases of severe grade III, IV, or even fatal neurotoxicity as well as cases of ascending myelopathy have also been reported with most of these cases being irreversible. In this article, we report a reversible grade IV Guillain-Barre-like case of a patient with primary refractory T-cell lymphoblastic lymphoma treated with Nelarabine. Guillain-Barre-like syndrome in this patient coexisted with toxic myelopathy which affected the whole spine. The pathogenetic mechanisms and genetic predisposition for Nelarabine-associated neurotoxicity is still unknown. The role of the immune system and the patient's genetic background are under investigation along with considerations on the right treatment of the syndrome. Gaining a better understanding in the contributing mechanisms will help us to recognize individuals in danger for neurotoxicity and will lead to the prompt treatment of this complication. Yet, it can be concluded from the present case and literature review that high-dose cytarabine regimens and intrathecal installations should be avoided in close time proximity with Nelarabine treatment, as they could enhance neurotoxicity.
Nelarabine in the treatment of pediatric and adult patients with T-cell acute lymphoblastic leukemia and lymphoma.[Pubmed:27869523]
Expert Rev Hematol. 2017 Jan;10(1):1-8.
INTRODUCTION: T-cell acute lymphoblastic leukemia (ALL) and lymphoma (LBL) are aggressive hematologic neoplasms that are treated with combination chemotherapy in the frontline, but have limited options in the relapsed or refractory setting. Based on observations in patients with purine nucleoside phosphorylase (PNP) deficiency, a guanosine nucleoside analogue, arabinosylguanine (ara-G) was developed that provided T-cell specificity. Nelarabine was developed as the water-soluble, clinically useful-prodrug of ara-G and based on its activity was approved for the treatment of relapsed or refractory T-ALL/LBL. Areas covered: In this narrative review, we will summarize the preclinical studies, early dose-finding studies, and efficacy studies that led to approval of Nelarabine. The review will succinctly cover response rates and safety signals reported during clinical development. We will also cover more recent work with Nelarabine, including combination studies, modified dosing schedules, and frontline treatment approaches. Expert commentary: Based on evidence from the literature review and our own experience with Nelarabine, we conclude that it is an effective agent in the treatment of T-cell malignancies. Understanding the factors that modulate the risk of dose-limiting neurotoxicity, how to mitigate this toxicity, and how to safely combine it with other active agents will continue to broaden its use.
Improving nelarabine efficacy in T cell acute lymphoblastic leukemia by targeting aberrant PI3K/AKT/mTOR signaling pathway.[Pubmed:27776559]
J Hematol Oncol. 2016 Oct 24;9(1):114.
BACKGROUND: Although in recent years, the introduction of novel chemotherapy protocols has improved the outcome of T cell acute lymphoblastic leukemia (T-ALL) patients, refractory and/or relapsing disease remains a foremost concern. In this context, a major contribution was provided by the introduction of the nucleoside analog Nelarabine, approved for salvage treatment of T-ALL patients with refractory/relapsed disease. However, Nelarabine could induce a life-threatening, dose-dependent neurotoxicity. To improve Nelarabine efficacy, we have analyzed its molecular targets, testing selective inhibitors of such targets in combination with Nelarabine. METHODS: The effectiveness of Nelarabine as single agent or in combination with PI3K, Bcl2, and MEK inhibitors was evaluated on human T-ALL cell lines and primary T-ALL refractory/relapsed lymphoblasts. The efficacy of signal modulators in terms of cytotoxicity, induction of apoptosis, and changes in gene and protein expression was assessed by flow cytometry, western blotting, and quantitative real-time PCR in T-ALL settings. RESULTS: Treatment with Nelarabine as a single agent identified two groups of T-ALL cell lines, one sensitive and one resistant to the drug. Whereas sensitive T-ALL cells showed a significant increase of apoptosis and a strong down-modulation of PI3K signaling, resistant T-ALL cells showed a hyperactivation of AKT and MEK/ERK1/2 signaling pathways, not caused by differences in the expression of Nelarabine transporters or metabolic activators. We then studied the combination of Nelarabine with the PI3K inhibitors (both pan and dual gamma/delta inhibitors), with the Bcl2 specific inhibitor ABT199, and with the MEK inhibitor trametinib on both T-ALL cell lines and patient samples at relapse, which displayed constitutive activation of PI3K signaling and resistance to Nelarabine alone. The combination with the pan PI3K inhibitor ZSTK-474 was the most effective in inhibiting the growth of T-ALL cells and was synergistic in decreasing cell survival and inducing apoptosis in Nelarabine-resistant T-ALL cells. The drug combination caused AKT dephosphorylation and a downregulation of Bcl2, while Nelarabine alone induced an increase in p-AKT and Bcl2 signaling in the resistant T-ALL cells and relapsed patient samples. CONCLUSIONS: These findings indicate that Nelarabine in combination with PI3K inhibitors may be a promising therapeutic strategy for the treatment of T-ALL relapsed patients.
Gene expression ratio as a predictive determinant of nelarabine chemosensitivity in T-lymphoblastic leukemia/lymphoma.[Pubmed:27576612]
Pediatr Blood Cancer. 2017 Feb;64(2):250-253.
BACKGROUND: Nelarabine has been used for the treatment of T-cell malignancies including T-acute lymphoblastic leukemia (T-ALL)/T-lymphoblastic lymphoma. However, the mechanisms that underlie the susceptibility or resistance to Nelarabine have not been fully elucidated. The aim of this study was to determine the significance of Nelarabine transport and metabolism in the context of Nelarabine cytotoxicity. PROCEDURE: The expression profiles of six genes in the Nelarabine pathway were analyzed in blast cells from six patients with T-ALL as well as in three T-ALL cell lines. In vitro cytotoxicity (LC50 of 9-beta-d-arabinofuranosylguanine [ara-G]) was evaluated. RESULTS: The mRNA expression of ENT1, DCK, CDA, NT5C2, RRM1, and RRM2 in patients showed inter-individual variability and was not correlated with the LC50 of ara-G. However, the ratio of (ENT1 x DCK)/(CDA x RRM1) expression was significantly correlated with LC50 (r = -0.831, P = 0.0405). CONCLUSIONS: Chemosensitivity to Nelarabine is influenced by the balance of the expression of these four genes, and the ratio of their expression predicts the response of T-cell malignancies to Nelarabine.


