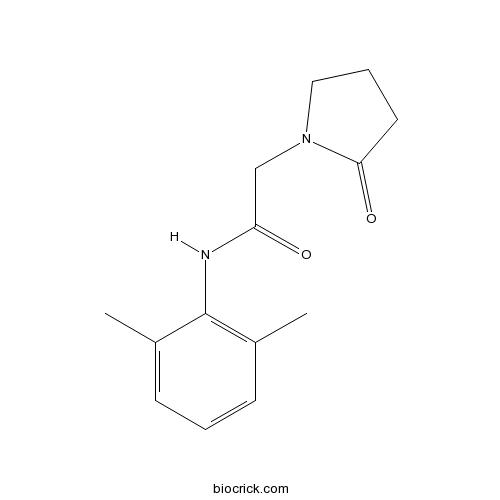
NefiracetamCognitive enhancer CAS# 77191-36-7 |
- Dexmedetomidine HCl
Catalog No.:BCC4347
CAS No.:145108-58-3
- Xylazine HCl
Catalog No.:BCC4341
CAS No.:23076-35-9
- Guanfacine
Catalog No.:BCC5180
CAS No.:29110-47-2
- Sotalol
Catalog No.:BCC4356
CAS No.:3930-20-9
- Isoprenaline HCl
Catalog No.:BCC4328
CAS No.:51-30-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 77191-36-7 | SDF | Download SDF |
| PubChem ID | 71157 | Appearance | Powder |
| Formula | C14H18N2O2 | M.Wt | 246.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | DM 9384 | ||
| Solubility | DMSO : ≥ 100 mg/mL (406.01 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | N-(2,6-dimethylphenyl)-2-(2-oxopyrrolidin-1-yl)acetamide | ||
| SMILES | CC1=C(C(=CC=C1)C)NC(=O)CN2CCCC2=O | ||
| Standard InChIKey | NGHTXZCKLWZPGK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H18N2O2/c1-10-5-3-6-11(2)14(10)15-12(17)9-16-8-4-7-13(16)18/h3,5-6H,4,7-9H2,1-2H3,(H,15,17) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cognitive enhancer that displays various pharmacological effects. Activates L/N-type calcium channels, cholinergic, monoaminergic and GABAergic systems. Displays potent neuroprotective action in the retinal ischemia-reperfusion model in vivo. |

Nefiracetam Dilution Calculator

Nefiracetam Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0601 mL | 20.3004 mL | 40.6009 mL | 81.2018 mL | 101.5022 mL |
| 5 mM | 0.812 mL | 4.0601 mL | 8.1202 mL | 16.2404 mL | 20.3004 mL |
| 10 mM | 0.406 mL | 2.03 mL | 4.0601 mL | 8.1202 mL | 10.1502 mL |
| 50 mM | 0.0812 mL | 0.406 mL | 0.812 mL | 1.624 mL | 2.03 mL |
| 100 mM | 0.0406 mL | 0.203 mL | 0.406 mL | 0.812 mL | 1.015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Nefiracetam is a GABAergic, cholinergic, and monoaminergic neuronal systems enhancer for Ro 5-4864-induced convulsions. Phase 2.
- Rheediaxanthone A
Catalog No.:BCN7411
CAS No.:77181-97-6
- Acetylaconitine
Catalog No.:BCN2407
CAS No.:77181-26-1
- Delta-Caesalpin
Catalog No.:BCN6698
CAS No.:7716-14-5
- Spiraline
Catalog No.:BCN2112
CAS No.:77156-25-3
- Spiracine
Catalog No.:BCN2095
CAS No.:77156-24-2
- Spiranine
Catalog No.:BCN2094
CAS No.:77156-23-1
- 6alpha-Hydroxycleroda-3,13-dien-16,15-olid-18-oic acid
Catalog No.:BCN1359
CAS No.:771493-42-6
- SR 57227 hydrochloride
Catalog No.:BCC6967
CAS No.:77145-61-0
- Fmoc-N-Me-Phe-OH
Catalog No.:BCC2614
CAS No.:77128-73-5
- Fmoc-Phe(4-OMe)-OH,Fmoc-Tyr(Me)-OH
Catalog No.:BCC2634
CAS No.:77128-72-4
- Fmoc-Sar-OH
Catalog No.:BCC3338
CAS No.:77128-70-2
- 1H-Indole-3-carboxylic acid
Catalog No.:BCN4324
CAS No.:771-50-6
- Carboxyatractyloside
Catalog No.:BCN2880
CAS No.:77228-71-8
- MG 624
Catalog No.:BCC7028
CAS No.:77257-42-2
- Erythrabyssin II
Catalog No.:BCN4828
CAS No.:77263-06-0
- Abyssinone V
Catalog No.:BCN6825
CAS No.:77263-11-7
- Zederone
Catalog No.:BCN3524
CAS No.:7727-79-9
- Fmoc-Nle-OH.
Catalog No.:BCC3298
CAS No.:77284-32-3
- Edelfosine
Catalog No.:BCC7537
CAS No.:77286-66-9
- Hirsutine
Catalog No.:BCN2758
CAS No.:7729-23-9
- SL 0101-1
Catalog No.:BCC8086
CAS No.:77307-50-7
- Hyuganin D
Catalog No.:BCN7679
CAS No.:77331-76-1
- Acamprosate calcium
Catalog No.:BCC1327
CAS No.:77337-73-6
- Triacetylpseurotin A
Catalog No.:BCN6916
CAS No.:77353-57-2
Molecular dynamics study-based mechanism of nefiracetam-induced NMDA receptor potentiation.[Pubmed:25659913]
Comput Biol Chem. 2015 Apr;55:14-22.
Plastic changes in the brain required for memory formation and long-term learning are dependent on N-methyl-d-aspartic acid (NMDA) receptor signaling. Nefiracetam reportedly boosts NMDA receptor functions as a basis for its nootropic properties. Previous studies suggest that Nefiracetam potentiates the NMDA receptor activation, as a more potent co-agonist for glycine binding site than glycine, though the underlying mechanisms remain elusive. Here, using BSP-SLIM method, a novel binding site within the core of spiral beta-strands-1-5 of LBD-GLUN1 has been predicted in glycine-bound GLUN1 conformation in addition to the glycine pocket in Apo-GLUN1. Within the core of spiral beta-strands-1-5 of LBD-GLUN1 pocket, all-atom molecular dynamics simulation revealed that Nefiracetam disrupts Arg523-glycine-Asp732 interaction resulting in open GLUN1 conformation and ultimate diffusion of glycine out of the clamshell cleft. Open GLUN1 conformation coerces other intra-chain domains and proximal inter-chain domains to sample inactivate conformations resulting in closure of the transmembrane gate via a novel gauche trap on threonine 647 (chi-1 dihedral (chi1)=-45 degrees instead of +45 degrees ). Docking of Nefiracetam into the glycine pocket reversed the gauche trap and meditates partial opening of the TMD gate within a time-scale of 100ns as observed in glycine-only state. All these results suggest that Nefiracetam can favorably complete with glycine for GLUN1-LBD in a two-step process, first by binding to a novel site of GLUN1-LBD-NMDA receptor followed by disruption of glycine-binding dynamics then replacing glycine in the GLUN1-LBD cleft.
A Randomized, Placebo-Controlled, Double-Blind Efficacy Study of Nefiracetam to Treat Poststroke Apathy.[Pubmed:26915605]
J Stroke Cerebrovasc Dis. 2016 May;25(5):1119-1127.
BACKGROUND: To evaluate the efficacy of treatment with Nefiracetam compared to placebo in poststroke apathy. METHODS: A parallel group, randomized, placebo-controlled, double-blind two-center trial in patients with recent stroke and apathy was conducted in 2 tertiary teaching hospitals in Perth, Western Australia, between March 2010 and October 2014. Consenting patients hospitalized with stroke were screened for participation at the time of hospitalization and, if diagnosed with apathy 8-36 weeks later, they were randomized to 12 weeks of 900 mg/day Nefiracetam or placebo. The primary efficacy parameter was change in apathy at 12 weeks defined by the 14-item Apathy Scale (AS). RESULTS: Of 2514 patients screened, only 377 (15%) were eligible for the study after the first screening, 233 declined further participation, and 144 were assessed for apathy at 8-36 weeks post stroke to confirm eligibility. Twenty patients out of 106 with a complete psychiatric assessment had apathy (19%). Of this sample, 13 patients were randomized. Overall, the AS score decreased by a mean of 7.0 points (95% CI = -14.6 to .6), but there was no significant between-group difference at week 12 (mean paired t-tests, P > .14). CONCLUSIONS: Treatment with Nefiracetam did not prove to be more efficacious than placebo in ameliorating apathy in stroke. The main limitation was the very small sample randomized, highlighting the limitations of conducting drug trials for behavioral problems among stroke patients. Pharmacological studies of apathy in stroke will require a large multicenter study and a massive sample of patients.
Nefiracetam Attenuates Pro-Inflammatory Cytokines and GABA Transporter in Specific Brain Regions of Rats with Post-Ischemic Seizures.[Pubmed:26584300]
Cell Physiol Biochem. 2015;37(5):2023-31.
BACKGROUND/AIMS: Prior studies demonstrated that pro-inflammatory cytokines (PICs) including IL-1beta, IL-6 and TNF-alpha contribute to regulation of epilepsy-associated pathophysiological processes in the specific brain regions, namely the parietal cortex, hippocampus and amygdala. Moreover, GABA transporter type 1 and 3 (GAT-1 and GAT-3) modulating extracellular GABA levels are engaged in the role played by PICs in epileptogenesis. Note that brain ischemic injury also elevates cerebral PICs. Thus, in this report we examined the effects of Nefiracetam (NEF), a pyrrolidone derivative, on the levels of IL-1beta, IL-6 and TNF-alpha, and expression of GAT-1 and GAT-3 in the parietal cortex, hippocampus and amygdala using a rat model of post-ischemic nonconvulsive seizure (NCS). METHODS: NCS was evoked by the middle cerebral artery occlusion (MCAO). ELISA and Western Blot analysis were employed to determine the levels of PICs and GAT-1/GAT-3, respectively. RESULTS: MCAO significantly increased IL-1beta, IL-6 and TNF-alpha in the parietal cortex, hippocampus and amygdala as compared with sham control animals (P<0.05 vs. control rats). Also, in these specific brain regions expression of GAT-1 and GAT-3 was amplified; and the levels of GABA were decreased in rats following MCAO (P<0.05 vs. control rats). Systemic administration of NEF significantly attenuated the elevated PICs, amplified GAT-1 and GAT-3 as well as impaired GABA. NEF also attenuated the number of NCS events following MCAO. CONCLUSION: our data demonstrate that NEF improves post-ischemia evoked-NCS by altering PICs, GABA transporters thereby alleviating GABA in the parietal cortex, hippocampus and amygdala. This support a role for PICs and GABA in engagement of the adaptive responses associated with epileptic activity, but also suggests that NEF has anti-epileptic effects via PICs-GABA mechanisms, having pharmacological implications to target the specific PICs for neuronal dysfunction and vulnerability related to post-ischemic seizures and cognitive impairment.
Nefiracetam attenuates post-ischemic nonconvulsive seizures in rats and protects neuronal cell death induced by veratridine and glutamate.[Pubmed:23603142]
Life Sci. 2013 Jun 13;92(22):1055-63.
AIMS: Stroke patients are at a high risk of developing post-ischemic seizures and cognitive impairment. Nefiracetam (NEF), a pyrrolidone derivative, has been shown to possess both anti-epileptic and cognitive-enhancing properties. In this study the anti-seizure effects of NEF were evaluated in a rat model of post-ischemic nonconvulsive seizures (NCSs). Its potential mechanisms were investigated in neuronal cell culture assays of neurotoxicity associated with ischemic brain injury and epileptogenesis. MAIN METHODS: In the in vivo study, rats received 24h permanent middle cerebral artery occlusion. NEF was administered intravenously either at 15 min post-injury but prior to the first NCS event (30 mg/kg, pre-NCS treatment) or immediately after the first NCS occurred (30 or 60 mg/kg, post-NCS treatment). In the in vitro study, neuronal cell cultures were exposed to veratridine or glutamate and treated with NEF (1-500 nM). KEY FINDINGS: The NEF pre-NCS treatment significantly reduced the NCS frequency and duration, whereas the higher NEF dose (60 mg/kg) was required to achieve similar effects when given after NCS occurred. The NEF treatment also dose-dependently (5-500 nM) protected against neuronal cell death induced by veratridine as measured by MTT cell viability assay, but higher doses (250-500 nM) were required against glutamate toxicity. SIGNIFICANCE: The anti-seizure property of NEF was demonstrated in a clinically relevant rat model of post-ischemic NCS. The preferential effects of NEF against in vitro veratridine toxicity suggest the involvement of its modulation of sodium channel malfunction. Future studies are warranted to study the mechanisms of NEF against ischemic brain injury and post-ischemic seizures.
Effects of Nefiracetam, a novel pyrrolidone-type nootropic agent, on the amygdala-kindled seizures in rats.[Pubmed:16190926]
Epilepsia. 2005 Oct;46(10):1561-8.
PURPOSE: Nefiracetam (NEF) is a novel pyrrolidonetype nootropic agent, and it has been reported to possess various pharmacologic effects as well as cognition-enhancing effects. The present study focused on the effects of NEF in amygdala-kindled seizures and its potential for antiepileptic therapy. METHODS: Effects of NEF on fully amygdala-kindled seizures and development of amygdala-kindled seizures were investigated in rats and compared with those of levetiracetam (LEV), a pyrrolidone-type antiepileptic drug (AED). RESULTS: In fully amygdala-kindled rats, NEF (25, 50, and 100 mg/kg, p.o.) decreased afterdischarge induction, afterdischarge duration, seizure stage, and motor seizure duration in a dose-dependent manner. LEV (25, 50, and 100 mg/kg, p.o.) had no effects on afterdischarge induction and slightly decreased afterdischarge duration, whereas it markedly decreased seizure stage and motor seizure duration. In contrast to the results in fully amygdala-kindled rats, NEF (25 and 50 mg/kg/day, p.o.) had few or no effects on the development of amygdala-kindled seizures. As well as fully amygdala-kindled seizures, LEV (50 mg/kg/day, p.o.) markedly inhibited the development of behavioral seizures without reducing daily afterdischarge duration. CONCLUSIONS: Although NEF possesses potent anticonvulsant effects on fully amygdala-kindled seizures, it has few or no effects on the development of amygdala-kindled seizures. LEV shows marked anticonvulsant effects on both phases of kindling. In fully amygdala-kindled rats, NEF inhibits both electroencephalographic and behavioral seizures, whereas LEV inhibits only behavioral seizures. This double dissociation suggests that NEF has a distinct anticonvulsant spectrum and mechanisms from those of LEV.
Mechanisms of action of cognitive enhancers on neuroreceptors.[Pubmed:15516710]
Biol Pharm Bull. 2004 Nov;27(11):1701-6.
No strategies for curing Alzheimer's disease have been developed yet as we do not know the exact cause of the disease. The only therapy that is available for patients is symptomatic treatment. Since Alzheimer's disease is associated with downregulation of the cholinergic system in the brain, its stimulation is expected to improve the patients' cognition, learning, and memory. Four anticholinesterases have been approved in the U.S.A. for the treatment of Alzheimer's disease patients. However, because of the inhibition of cholinesterases, these drugs have side effects and their effectiveness does not last long. Thus new approaches are needed. One approach is to stimulate directly nicotinic acetylcholine (nACh) receptors in the brain, and another is to stimulate NMDA receptors which are also known to be downregulated in Alzheimer's patients. Nefiracetam has been shown to potentiate ACh currents in the alpha4beta2 receptor of rat cortical neurons with a bell-shaped dose-response relationship and the maximum effect at 1 nM. This effect was exerted via G(s) proteins. The alpha7 receptor was almost unaffected by Nefiracetam. Nefiracetam also potentiated NMDA currents with the maximum effect at 10 nM via interaction with the glycine-binding site of the receptor. Galantamine had a moderate potentiating effect on the alpha4beta2 receptor and potentiated NMDA currents with the maximum effect at 1 microM. However, galantamine did not interact with the glycine-binding site. Donepezil, a potent anticholinesterase, also potentiated NMDA currents at 1-10000 nM. In conclusion, these three drugs potentiate the activity not only of the cholinergic system but also of the NMDA system, thereby stimulating the downregulated nACh receptors and NMDA receptors to improve patients' learning, cognition, and memory.
The cognition-enhancer nefiracetam inhibits both necrosis and apoptosis in retinal ischemic models in vitro and in vivo.[Pubmed:14718588]
J Pharmacol Exp Ther. 2004 Apr;309(1):200-7.
The retinal ischemic-reperfusion stress (130 mm Hg, 45 min) caused neuronal damage throughout all cell layers and reduced the thickness of retinal layer by 30% at 7 days after the stress of mouse retina. The intravitreous injection of 100 pmol of Nefiracetam, a cognition-enhancer, completely prevented the damage when it was given 30 min before and 3 h after the stress. Partial prevention was observed when it was given 24 h after the stress, or low dose (10 pmol) Nefiracetam was given 30 min before the stress. However, aniracetam had no effect. In the retinal cell line N18-RE-105, the ischemic-reperfusion stress by 2 h culture under the serum-free condition with low oxygen (less of 0.4% O(2)) and low glucose (1 mM) caused necrosis or apoptosis in the low-density (0.5 x 10(4) cell/cm(2))or high-density (5 x 10(4) cell/cm(2)) culture, respectively. The necrosis showed membrane disruption, loss of electron density, and mitochondrial swelling, whereas apoptosis showed nuclear fragmentation and condensation in transmission electron microscopical analyses and in experiments using specific cell death markers. Nefiracetam inhibited both necrosis and apoptosis, whereas brain-derived neurotrophic factor (BDNF) inhibited only apoptosis. The cell-protective actions of Nefiracetam were abolished by nifedipine and omega-conotoxin GVIA, L-type and N-type calcium channel blocker, but not by PD98059 or wortmannin, extracellular signal-regulated kinase 1/2 or phosphoinositide 3-kinase inhibitor, respectively, whereas those of BDNF were abolished by PD98059 and wortmannin, but not by nifedipine and omega-conotoxin GVIA. All these findings suggest that Nefiracetam inhibit necrosis and apoptosis occurred in the ischemic/hypoxic neuronal injury through an increase in Ca(2+) influx.


