Erythrabyssin IICAS# 77263-06-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 77263-06-0 | SDF | Download SDF |
| PubChem ID | 10408212 | Appearance | Powder |
| Formula | C25H28O4 | M.Wt | 392.5 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
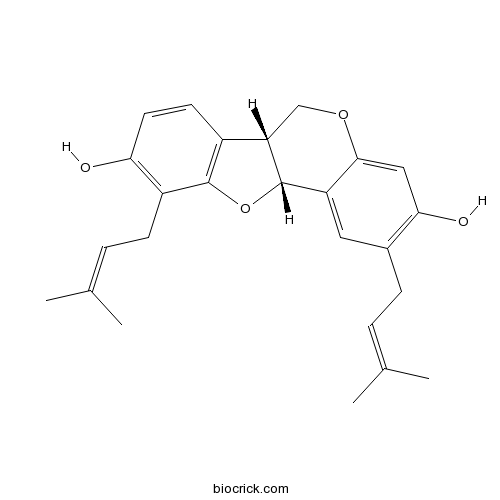
| Chemical Name | (6aR,11aR)-2,10-bis(3-methylbut-2-enyl)-6a,11a-dihydro-6H-[1]benzofuro[3,2-c]chromene-3,9-diol | ||
| SMILES | CC(=CCC1=C(C=C2C(=C1)C3C(CO2)C4=C(O3)C(=C(C=C4)O)CC=C(C)C)O)C | ||
| Standard InChIKey | LDKAMVCGTURXMH-CPJSRVTESA-N | ||
| Standard InChI | InChI=1S/C25H28O4/c1-14(2)5-7-16-11-19-23(12-22(16)27)28-13-20-17-9-10-21(26)18(8-6-15(3)4)24(17)29-25(19)20/h5-6,9-12,20,25-27H,7-8,13H2,1-4H3/t20-,25-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Erythrabyssin II actives against several strains of Staphylococcus and Streptococcus with an MIC range of 0.78-1.56 microg/ml. 2. Erythrabyssin II inhibits bacterial neuraminidase in a dose-dependent manner with significant inhibition (IC(50)=0.09-3.25 μM). |
| Targets | Antifection |

Erythrabyssin II Dilution Calculator

Erythrabyssin II Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5478 mL | 12.7389 mL | 25.4777 mL | 50.9554 mL | 63.6943 mL |
| 5 mM | 0.5096 mL | 2.5478 mL | 5.0955 mL | 10.1911 mL | 12.7389 mL |
| 10 mM | 0.2548 mL | 1.2739 mL | 2.5478 mL | 5.0955 mL | 6.3694 mL |
| 50 mM | 0.051 mL | 0.2548 mL | 0.5096 mL | 1.0191 mL | 1.2739 mL |
| 100 mM | 0.0255 mL | 0.1274 mL | 0.2548 mL | 0.5096 mL | 0.6369 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- MG 624
Catalog No.:BCC7028
CAS No.:77257-42-2
- Carboxyatractyloside
Catalog No.:BCN2880
CAS No.:77228-71-8
- Nefiracetam
Catalog No.:BCC4504
CAS No.:77191-36-7
- Rheediaxanthone A
Catalog No.:BCN7411
CAS No.:77181-97-6
- Acetylaconitine
Catalog No.:BCN2407
CAS No.:77181-26-1
- Delta-Caesalpin
Catalog No.:BCN6698
CAS No.:7716-14-5
- Spiraline
Catalog No.:BCN2112
CAS No.:77156-25-3
- Spiracine
Catalog No.:BCN2095
CAS No.:77156-24-2
- Spiranine
Catalog No.:BCN2094
CAS No.:77156-23-1
- 6alpha-Hydroxycleroda-3,13-dien-16,15-olid-18-oic acid
Catalog No.:BCN1359
CAS No.:771493-42-6
- SR 57227 hydrochloride
Catalog No.:BCC6967
CAS No.:77145-61-0
- Fmoc-N-Me-Phe-OH
Catalog No.:BCC2614
CAS No.:77128-73-5
- Abyssinone V
Catalog No.:BCN6825
CAS No.:77263-11-7
- Zederone
Catalog No.:BCN3524
CAS No.:7727-79-9
- Fmoc-Nle-OH.
Catalog No.:BCC3298
CAS No.:77284-32-3
- Edelfosine
Catalog No.:BCC7537
CAS No.:77286-66-9
- Hirsutine
Catalog No.:BCN2758
CAS No.:7729-23-9
- SL 0101-1
Catalog No.:BCC8086
CAS No.:77307-50-7
- Hyuganin D
Catalog No.:BCN7679
CAS No.:77331-76-1
- Acamprosate calcium
Catalog No.:BCC1327
CAS No.:77337-73-6
- Triacetylpseurotin A
Catalog No.:BCN6916
CAS No.:77353-57-2
- Drimiopsin C
Catalog No.:BCN4325
CAS No.:773850-90-1
- Drimiopsin D
Catalog No.:BCN4326
CAS No.:773850-91-2
- Sesartemin
Catalog No.:BCN4779
CAS No.:77394-27-5
Antibacterial pterocarpans from Erythrina subumbrans.[Pubmed:17055201]
J Ethnopharmacol. 2007 Mar 1;110(1):171-5.
Seven pterocarpans, erybraedin B (1), erybraedin A (2), phaseollin (3), Erythrabyssin II (4), erystagallin A (5), erythrabissin-1 (6) and erycristagallin (7), two flavanones, 5-hydroxysophoranone (8) and glabrol (9), and one isoflavone, erysubin F (10), were isolated from the stems of Erythrina subumbrans (Leguminosae). Their structures were identified by means of spectroscopy. This is the first report of the isolation of the non-alkaloidal compounds from Erythrina subumbrans and the observed dehydration of 6a-hydroxypterocarpans 5 and 6 in CDCl(3) to the corresponding pterocarpenes 11 and 12, respectively. Compounds 8 and 9 were isolated for the first time from the genus Erythrina. Compounds 2 and 4 exhibited the highest degree of activity against Streptococcus strains with an MIC range of 0.78-1.56 microg/ml, whereas compound 7 exhibited the highest degree of activity against Staphylococcus strains, including drug-resistant strains (MRSA and VRSA), with an MIC range of 0.39-1.56 microg/ml. Interestingly, compounds 2, 4, 5 and 7 were more active against several strains of Streptococcus and Staphylococcus than the standard antibiotics vancomycin and oxacillin. Compound 7 showed the highest level of activity against all VRSA strains tested, with an MIC range of 0.39-1.56 microg/ml, which were resistant to both antibiotics. These compounds may prove to be potent phytochemical agents for antibacterial activity, especially against the MRSA and VRSA strains.
Potent inhibition of bacterial neuraminidase activity by pterocarpans isolated from the roots of Lespedeza bicolor.[Pubmed:21911291]
Bioorg Med Chem Lett. 2011 Oct 15;21(20):6100-3.
Bacterial neuraminidase has been highlighted as a key enzyme for pathogenic infection and sepsis. Six pterocarpans displaying significant levels of neuraminidase inhibitory activity were isolated from the root bark of Lespedeza bicolor. The isolated compounds were identified as three new pterocarpans (1-3) together with known compounds Erythrabyssin II (4), lespebuergine G4 (5), and 1-methoxyErythrabyssin II (6). The new compounds were characterized as bicolosin A (1), bicolosin B (2), and bicolosin C (3). All compounds inhibited bacterial neuraminidase in a dose-dependent manner with significant inhibition (IC(50)=0.09-3.25 muM). All neuraminidase inhibitors screened were found to exhibit noncompetitive kinetics. The three most potent neuraminidase inhibitors (1, 3 and 6) feature a methoxy substitution on C-1.


