NKY 80Adenylyl cyclase inhibitor CAS# 299442-43-6 |
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 299442-43-6 | SDF | Download SDF |
| PubChem ID | 2772368 | Appearance | Powder |
| Formula | C12H11N3O2 | M.Wt | 229.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 10 mM in ethanol with gentle warming | ||
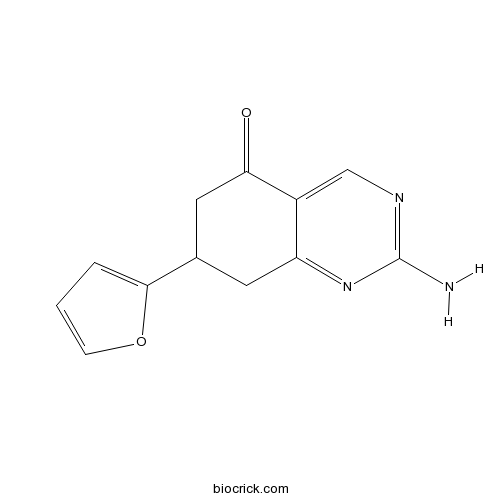
| Chemical Name | 2-amino-7-(furan-2-yl)-7,8-dihydro-6H-quinazolin-5-one | ||
| SMILES | C1C(CC(=O)C2=CN=C(N=C21)N)C3=CC=CO3 | ||
| Standard InChIKey | SOJUSNIBPPMLCC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H11N3O2/c13-12-14-6-8-9(15-12)4-7(5-10(8)16)11-2-1-3-17-11/h1-3,6-7H,4-5H2,(H2,13,14,15) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of adenylyl cyclase (AC). Exhibits greater affinity for AC5 over AC3 and AC2 (IC50 values are 8.3 μM, 132 μM and 1.7 mM respectively). |

NKY 80 Dilution Calculator

NKY 80 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3624 mL | 21.8122 mL | 43.6243 mL | 87.2486 mL | 109.0608 mL |
| 5 mM | 0.8725 mL | 4.3624 mL | 8.7249 mL | 17.4497 mL | 21.8122 mL |
| 10 mM | 0.4362 mL | 2.1812 mL | 4.3624 mL | 8.7249 mL | 10.9061 mL |
| 50 mM | 0.0872 mL | 0.4362 mL | 0.8725 mL | 1.745 mL | 2.1812 mL |
| 100 mM | 0.0436 mL | 0.2181 mL | 0.4362 mL | 0.8725 mL | 1.0906 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- NPS ALX Compound 4a dihydrochloride
Catalog No.:BCC7629
CAS No.:299433-10-6
- 5,6-Dimethyl-2-Benzothiazolamine
Catalog No.:BCC8723
CAS No.:29927-08-0
- Ro 20-1724
Catalog No.:BCC6638
CAS No.:29925-17-5
- 7-Hydroxy-6-methoxy-3-prenylcoumarin
Catalog No.:BCN5207
CAS No.:299159-90-3
- Eledoisin-Related Peptide
Catalog No.:BCC5849
CAS No.:2990-43-4
- Sparteine sulfate pentahydrate
Catalog No.:BCN1267
CAS No.:299-39-8
- Astringin
Catalog No.:BCN3412
CAS No.:29884-49-9
- Amygdalin
Catalog No.:BCN5206
CAS No.:29883-15-6
- Ro 67-7476
Catalog No.:BCC6145
CAS No.:298690-60-5
- Pirenzepine dihydrochloride
Catalog No.:BCC6923
CAS No.:29868-97-1
- Astilbin
Catalog No.:BCN5204
CAS No.:29838-67-3
- Shanzhiside
Catalog No.:BCN5203
CAS No.:29836-27-9
- 1,3,5-Trihydroxy-4-(3-hydroxy-3-methylbutyl)xanthone
Catalog No.:BCN1462
CAS No.:299895-11-7
- YM 202074
Catalog No.:BCC7682
CAS No.:299900-84-8
- Arecoline hydrobromide
Catalog No.:BCN2913
CAS No.:300-08-3
- H-Tyr(3,5-I2)-OH
Catalog No.:BCC3263
CAS No.:300-39-0
- Hypotaurine
Catalog No.:BCN1749
CAS No.:300-84-5
- Isoline
Catalog No.:BCN2063
CAS No.:30000-36-3
- HEAT hydrochloride
Catalog No.:BCC6683
CAS No.:30007-39-7
- Diphyllin O-glucoside
Catalog No.:BCN8065
CAS No.:30021-77-3
- Ro 28-1675
Catalog No.:BCC4124
CAS No.:300353-13-3
- Boc-Asn-ol
Catalog No.:BCC2587
CAS No.:30044-67-8
- Dehydrocorydalin
Catalog No.:BCN2474
CAS No.:30045-16-0
- SKF 77434 hydrobromide
Catalog No.:BCC7144
CAS No.:300561-58-4
Late INa increases diastolic SR-Ca2+-leak in atrial myocardium by activating PKA and CaMKII.[Pubmed:25990311]
Cardiovasc Res. 2015 Jul 1;107(1):184-96.
AIMS: Enhanced cardiac late Na current (late INa) and increased sarcoplasmic reticulum (SR)-Ca(2+)-leak are both highly arrhythmogenic. This study seeks to identify signalling pathways interconnecting late INa and SR-Ca(2+)-leak in atrial cardiomyocytes (CMs). METHODS AND RESULTS: In murine atrial CMs, SR-Ca(2+)-leak was increased by the late INa enhancer Anemonia sulcata toxin II (ATX-II). An inhibition of Ca(2+)/calmodulin-dependent protein kinase II (Autocamide-2-related inhibitory peptide), protein kinase A (H89), or late INa (Ranolazine or Tetrodotoxin) all prevented ATX-II-dependent SR-Ca(2+)-leak. The SR-Ca(2+)-leak induction by ATX-II was not detected when either the Na(+)/Ca(2+) exchanger was inhibited (KBR) or in CaMKIIdeltac-knockout mice. FRET measurements revealed increased cAMP levels upon ATX-II stimulation, which could be prevented by inhibition of adenylyl cyclases (ACs) 5 and 6 (NKY 80) but not by inhibition of phosphodiesterases (IBMX), suggesting PKA activation via an AC-dependent increase of cAMP levels. Western blots showed late INa-dependent hyperphosphorylation of CaMKII as well as PKA target sites at ryanodine receptor type-2 (-S2814 and -S2808) and phospholamban (-Thr17, -S16). Enhancement of late INa did not alter Ca(2+)-transient amplitude or SR-Ca(2+)-load. However, upon late INa activation and simultaneous CaMKII inhibition, Ca(2+)-transient amplitude and SR-Ca(2+)-load were increased, whereas PKA inhibition reduced Ca(2+)-transient amplitude and load and additionally slowed Ca(2+) elimination. In atrial CMs from patients with atrial fibrillation, inhibition of late INa, CaMKII, or PKA reduced the SR-Ca(2+)-leak. CONCLUSION: Late INa exerts distinct effects on Ca(2+) homeostasis in atrial myocardium through activation of CaMKII and PKA. Inhibition of late INa represents a potential approach to attenuate CaMKII activation and decreases SR-Ca(2+)-leak in atrial rhythm disorders. The interconnection with the cAMP/PKA system further increases the antiarrhythmic potential of late INa inhibition.
Capturing adenylyl cyclases as potential drug targets.[Pubmed:19337273]
Nat Rev Drug Discov. 2009 Apr;8(4):321-35.
Cyclic AMP (cAMP) is an important intracellular signalling mediator. It is generated in mammals by nine membrane-bound and one soluble adenylyl cyclases (ACs), each with distinct regulation and expression patterns. Although many drugs inhibit or stimulate AC activity through the respective upstream G-protein coupled receptors (for example, opioid or beta-adrenergic receptors), ACs themselves have not been major drug targets. Over the past decade studies on the physiological functions of the different mammalian AC isoforms as well as advances in the development of isoform-selective AC inhibitors and activators suggest that ACs could be useful drug targets. Here we discuss the therapeutic potential of isoform-selective compounds in various clinical settings, including neuropathic pain, neurodegenerative disorders, congestive heart failure, asthma and male contraception.
Type-specific regulation of adenylyl cyclase. Selective pharmacological stimulation and inhibition of adenylyl cyclase isoforms.[Pubmed:11602596]
J Biol Chem. 2001 Dec 21;276(51):47785-93.
Crystallographic studies have elucidated the binding mechanism of forskolin and P-site inhibitors to adenylyl cyclase. Accordingly, computer-assisted drug design has enabled us to identify isoform-selective regulators of adenylyl cyclase. After examining more than 200 newly synthesized derivatives of forskolin, we found that the modification at the positions of C6 and C7, in general, enhances isoform selectivity. The 6-(3-dimethylaminopropionyl) modification led to an enhanced selectivity for type V, whereas 6-[N-(2-isothiocyanatoethyl) aminocarbonyl] and 6-(4-acrylbutyryl) modification led to an enhanced selectivity for type II. In contrast, 2'-deoxyadenosine 3'-monophosphate, a classical and 3'-phosphate-substituted P-site inhibitor, demonstrated a 27-fold selectivity for inhibiting type V relative to type II, whereas 9-(tetrahydro-2-furyl) adenine, a ribose-substituted P-site ligand, showed a markedly increased, 130-fold selectivity for inhibiting type V. Consequently, on the basis of the pharmacophore analysis of 9-(tetrahydro-2-furyl) adenine and adenylyl cyclase, a novel non-nucleoside inhibitor, 2-amino-7-(2-furanyl)-7,8-dihydro-5(6H)-quinazolinone (NKY80), was identified after virtual screening of more than 850,000 compounds. NKY80 demonstrated a 210-fold selectivity for inhibiting type V relative to type II. More importantly, the combination of a type III-selective forskolin derivative and 9-(tetrahydro-2-furyl) adenine or NKY80 demonstrated a further enhanced selectivity for type III stimulation over other isoforms. Our data suggest the feasibility of adenylyl cyclase isoform-targeted regulation of cyclic AMP signaling by pharmacological reagents, either alone or in combination.


