Momordicine IICAS# 91590-75-9 |
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 91590-75-9 | SDF | Download SDF |
| PubChem ID | 131751889 | Appearance | Powder |
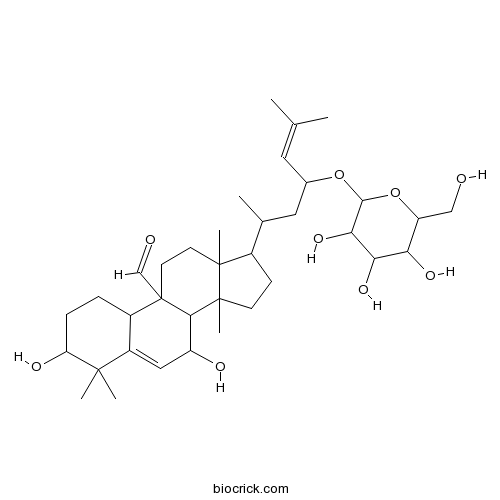
| Formula | C36H58O9 | M.Wt | 634.9 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Synonyms | Momordicin II | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,7-dihydroxy-4,4,13,14-tetramethyl-17-[6-methyl-4-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyhept-5-en-2-yl]-2,3,7,8,10,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthrene-9-carbaldehyde | ||
| SMILES | CC(CC(C=C(C)C)OC1C(C(C(C(O1)CO)O)O)O)C2CCC3(C2(CCC4(C3C(C=C5C4CCC(C5(C)C)O)O)C=O)C)C | ||
| Standard InChIKey | WCYLDCDQWJYEPO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C36H58O9/c1-19(2)14-21(44-32-30(43)29(42)28(41)26(17-37)45-32)15-20(3)22-10-11-35(7)31-25(39)16-24-23(8-9-27(40)33(24,4)5)36(31,18-38)13-12-34(22,35)6/h14,16,18,20-23,25-32,37,39-43H,8-13,15,17H2,1-7H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Momordicine II may have antidiabetic activity, it significantly stimulated insulin secretion. It also can deter oviposition by L. trifolii significantly. |
| In vitro | Saponins from the traditional medicinal plant Momordica charantia stimulate insulin secretion in vitro.[Pubmed: 22133295 ]Phytomedicine. 2011 Dec 15;19(1):32-7.The antidiabetic activity of Momordica charantia (L.), Cucurbitaceae, a widely-used treatment for diabetes in a number of traditional medicine systems, was investigated in vitro. Antidiabetic activity has been reported for certain saponins isolated from M. charantia. Cucurbitane glucosides from Momordica charantia leaves as oviposition deterrents to the leafminer, Liriomyza trifolii.[Pubmed: 16610222 ]Z Naturforsch C. 2006 Jan-Feb;61(1-2):81-6.
|
| Cell Research | Transport in Caco-2 cell monolayers of antidiabetic cucurbitane triterpenoids from Momordica charantia fruits.[Pubmed: 25116119 ]Planta Med. 2014 Jul;80(11):907-11.Bitter melon, the fruit of Momordica charantia L. (Cucurbitaceae), is a widely-used treatment for diabetes in traditional medicine systems throughout the world. Various compounds have been shown to be responsible for this reputed activity, and, in particular, cucurbitane triterpenoids are thought to play a significant role. The objective of this study was to investigate the gastrointestinal transport of a triterpenoid-enriched n-butanol extract of M. charantia using a two-compartment transwell human intestinal epithelial cell Caco-2 monolayer system, simulating the intestinal barrier. |

Momordicine II Dilution Calculator

Momordicine II Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5751 mL | 7.8753 mL | 15.7505 mL | 31.501 mL | 39.3763 mL |
| 5 mM | 0.315 mL | 1.5751 mL | 3.1501 mL | 6.3002 mL | 7.8753 mL |
| 10 mM | 0.1575 mL | 0.7875 mL | 1.5751 mL | 3.1501 mL | 3.9376 mL |
| 50 mM | 0.0315 mL | 0.1575 mL | 0.315 mL | 0.63 mL | 0.7875 mL |
| 100 mM | 0.0158 mL | 0.0788 mL | 0.1575 mL | 0.315 mL | 0.3938 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Quercetin 3-Caffeylrobinobioside
Catalog No.:BCN8744
CAS No.:957110-26-8
- Tessaric acid
Catalog No.:BCN8743
CAS No.:58142-10-2
- Ganoderic acid R
Catalog No.:BCN8489
CAS No.:103963-39-9
- L-Fucitol
Catalog No.:BCN8464
CAS No.:13074-06-1
- 1,3,6-Trihydroxy-2-methylanthraquinone 3-O-alpha-L-rhamnosyl-(1->2)-beta-D-glucoside
Catalog No.:BCN8732
CAS No.:87686-88-2
- Niga-ichigoside F1
Catalog No.:BCN8356
CAS No.:95262-48-9
- Cistantubuloside C1
Catalog No.:BCN6362
CAS No.:620632-36-2
- Isosilybin A
Catalog No.:BCN6369
CAS No.:142796-21-2
- Luteolin-3',7-di-O-glucoside
Catalog No.:BCN6380
CAS No.:257-724-7
- Isosilybin B
Catalog No.:BCN6764
CAS No.:142796-22-3
- Arecaidine
Catalog No.:BCN8282
CAS No.:499-04-7
- Xanthoangelol
Catalog No.:BCN8741
CAS No.:62949-76-2
- Polygalasaponin XXXV
Catalog No.:BCN8746
CAS No.:184479-28-5
- Marginatoxin
Catalog No.:BCN8747
CAS No.:1422536-56-8
- Leachianol G
Catalog No.:BCN8748
CAS No.:164204-62-0
- Atractylochromene
Catalog No.:BCN8749
CAS No.:203443-33-8
- Cassiaglycoside II
Catalog No.:BCN8750
CAS No.:2241081-56-9
- Momordicine V
Catalog No.:BCN8751
CAS No.:1012315-36-4
- Pueroside A
Catalog No.:BCN8752
CAS No.:100692-52-2
- Leachianol F
Catalog No.:BCN8753
CAS No.:164123-50-6
- Polygalasaponin E
Catalog No.:BCN8754
CAS No.:882664-72-4
- Yuanhuanin
Catalog No.:BCN8756
CAS No.:83133-14-6
- Ampelopsin G
Catalog No.:BCN8757
CAS No.:151487-09-1
- Torosachrysone 8-O-beta-gentiobioside
Catalog No.:BCN8758
CAS No.:94356-13-5
Saponins from the traditional medicinal plant Momordica charantia stimulate insulin secretion in vitro.[Pubmed:22133295]
Phytomedicine. 2011 Dec 15;19(1):32-7.
The antidiabetic activity of Momordica charantia (L.), Cucurbitaceae, a widely-used treatment for diabetes in a number of traditional medicine systems, was investigated in vitro. Antidiabetic activity has been reported for certain saponins isolated from M. charantia. In this study insulin secretion was measured in MIN6 beta-cells incubated with an ethanol extract, saponin-rich fraction, and five purified saponins and cucurbitane triterpenoids from M. charantia, 3beta,7beta,25-trihydroxycucurbita-5,23(E)-dien-19-al (1), momordicine I (2), Momordicine II (3), 3-hydroxycucurbita-5,24-dien-19-al-7,23-di-O-beta-glucopyranoside (4), and kuguaglycoside G (5). Treatments were compared to incubation with high glucose (27 mM) and the insulin secretagogue, glipizide (50 muM). At 125 mug/ml, an LC-ToF-MS characterized saponin-rich fraction stimulated insulin secretion significantly more than the DMSO vehicle, p=0.02. At concentrations 10 and 25 mug/ml, compounds 3 and 5 also significantly stimulated insulin secretion as compared to the vehicle, p
Cucurbitane-type triterpenoids from Momordica charantia.[Pubmed:20379957]
Planta Med. 2010 Oct;76(15):1758-61.
One new cucurbitane-type triterpenoid glycoside, momordicoside U (1), together with five known cucurbitane-type triterpenoids and related glycosides, 3beta,7 beta,25-trihydroxycucurbita-5,23 (E)-dien-19-al (2), momordicine I (3), Momordicine II (4), 3-hydroxycucurbita-5,24-dien-19-al-7,23-di-O-beta-glucopyranoside (5), and kuguaglycoside G (6), were isolated from the whole plant of Momordica charantia. Their structures were determined by chemical and spectroscopic methods. Momordicoside U (1) was evaluated for insulin secretion activity in an in vitro insulin secretion assay and displayed moderate activity.
A new oviposition deterrent to the leafminer, Liriomyza trifolii: cucurbitane glucoside from Momordica charantia.[Pubmed:17913080]
Z Naturforsch C J Biosci. 2007 Jul-Aug;62(7-8):603-7.
A new cucurbitane glucoside, 23-O-beta-D-glucopyranosyl-7-hydroxy-3-O-malonylcucurbita-5,24-dien-19-al, named momordicine V, has been isolated from Momordica charantia leaves, along with the previously reported compounds, momordicines I, II, IV and 3-O-malonylmomordicine I. The structure of the new compound was established on the basis of spectral analysis, as well as by its conversion to Momordicine II by alkaline catalyzed hydrolysis. Momordicine V deterred significantly the oviposition by L. trifolii on host plant leaves treated at 26.16 microg/cm2 leaf surface.
Cucurbitane glucosides from Momordica charantia leaves as oviposition deterrents to the leafminer, Liriomyza trifolii.[Pubmed:16610222]
Z Naturforsch C J Biosci. 2006 Jan-Feb;61(1-2):81-6.
The American serpentine leaf mining fly, Liriomyza trifolii, whose larva feeds on more than 120 plant species is well characterized by its high degree of polyphagy. Observations on the oviposition behavior by L. trifolii demonstrated that among cucurbitaceous plants, Momordica charantia is rarely attacked by L. trifolii. The methanol extract of M. charantia leaves strongly deterred the females from ovipositing on kidney bean leaves treated at a concentration of 1 g leaf equivalent extract/ml. Analysis of the polar fraction of the methanol extract of M. charantia leaves resulted in the isolation of a novel cucurbitane glucoside, 7-O-beta-D-glucopyranosyl-3,23-dihydroxycucurbita-5,24-dien-19-al, named momordicine IV, along with another known compound, Momordicine II. Momordicine II and IV deterred oviposition by L. trifolii significantly when bioassays were carried out on kidney bean leaves treated at 75.6 and 20.3 microg/cm2 leaf surface, respectively. There was no synergistic effect on oviposition deterrent when the two compounds were combined in their natural abundance.
Identification of triterpenoid feeding deterrent of red pumpkin beetles (Aulacophora foveicollis) fromMomordica charantia.[Pubmed:24302337]
J Chem Ecol. 1987 Jul;13(7):1689-94.
The triterpenoids isolated from the leaves ofMomordica charantia Linn (bitter gourd) were found to elicit feeding-deterrent activity against red pumpkin beetles (Aulacophora foveicollis Lucas). The most abundant triterpenoid which deterred feeding was identified as Momordicine II, 23-O-beta-glucopyranoside of 3,7,23-trihydroxycucurbita-5,24-dien-19-al. A concentration of 3200mug/ml and above of the triterpenoids caused significant reduction of feeding by red pumpkin beetles in in vitro bioassay experiments, which compared favorably with the levels of triterpenoids inM. charantia leaves found in nature.


