ArecaidineCAS# 499-04-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 499-04-7 | SDF | Download SDF |
| PubChem ID | 10355 | Appearance | Powder |
| Formula | C7H11NO2 | M.Wt | 141.17 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
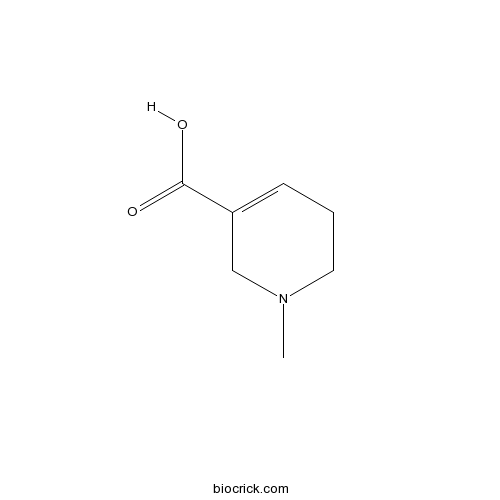
| Chemical Name | 1-methyl-3,6-dihydro-2~{H}-pyridine-5-carboxylic acid | ||
| SMILES | CN1CCC=C(C1)C(=O)O | ||
| Standard InChIKey | DNJFTXKSFAMXQF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H11NO2/c1-8-4-2-3-6(5-8)7(9)10/h3H,2,4-5H2,1H3,(H,9,10) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Arecaidine has tumorgenicity. |
| In vivo | Effects of the Areca nut constituents arecaidine and guvacine on the action of GABA in the cat central nervous system.[Pubmed: 922499 ]Brain Res. 1977 Nov 18;136(3):513-22.Arecaidine and guvacine, constituents of the nut of Areca catechu, inhibited the uptake of GABA and beta-alanine, but not that of glycine, by slices of cat spinal cord.
Transport of the areca nut alkaloid arecaidine by the human proton-coupled amino acid transporter 1 (hPAT1).[Pubmed: 23488788 ]J Pharm Pharmacol. 2013 Apr;65(4):582-90.The pyridine alkaloid Arecaidine is an ingredient of areca nut preparations. It is responsible for many physiological effects observed during areca nut chewing. However, the mechanism underlying its oral bioavailability has not yet been studied. We investigated whether the H⁺-coupled amino acid transporter 1 (PAT1, SLC36A1), which is expressed in the intestinal epithelium, accepts Arecaidine, arecoline, isoguvacine and other derivatives as substrates.
|
| Animal Research | Diffusion of reduced arecoline and arecaidine through human vaginal and buccal mucosa.[Pubmed: 8667258 ]J Oral Pathol Med. 1996 Feb;25(2):65-8.The purpose of the present study was to determine the minimal Arecaidine concentrations showing a synergistic effect on DMBA-induced hamster cheek pouch carcinogenesis.
|

Arecaidine Dilution Calculator

Arecaidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.0837 mL | 35.4183 mL | 70.8366 mL | 141.6732 mL | 177.0915 mL |
| 5 mM | 1.4167 mL | 7.0837 mL | 14.1673 mL | 28.3346 mL | 35.4183 mL |
| 10 mM | 0.7084 mL | 3.5418 mL | 7.0837 mL | 14.1673 mL | 17.7091 mL |
| 50 mM | 0.1417 mL | 0.7084 mL | 1.4167 mL | 2.8335 mL | 3.5418 mL |
| 100 mM | 0.0708 mL | 0.3542 mL | 0.7084 mL | 1.4167 mL | 1.7709 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Xanthoangelol
Catalog No.:BCN8741
CAS No.:62949-76-2
- Trillikamtoside Q
Catalog No.:BCN8195
CAS No.:2098642-70-5
- 2''-O-acetylsaikosaponin A
Catalog No.:BCN8736
CAS No.:102934-42-9
- (R)-alpha-methyltryptamine
Catalog No.:BCN8160
CAS No.:7795-52-0
- Quercetin 3-O-(6''-galloyl)-beta-D-glucopyranoside
Catalog No.:BCN8742
CAS No.:56316-75-7
- Scutellarein-7-O-glucoside
Catalog No.:BCN8099
CAS No.:26046-94-6
- Steviol-19-O-Glucoside
Catalog No.:BCN6373
CAS No.:1185737-16-9
- (-)-Epiafzelechin 3-O-gallate
Catalog No.:BCN8740
CAS No.:108907-43-3
- 8-Hydroxypinoresinol diglucoside
Catalog No.:BCN6368
CAS No.:112747-99-6
- Caraganaphenol A
Catalog No.:BCN8731
CAS No.:174916-31-5
- Cyclocurcumin
Catalog No.:BCN6379
CAS No.:153127-42-5
- Daidzein-4',7-diglucoside
Catalog No.:BCN8135
CAS No.:53681-67-7
- Isosilybin B
Catalog No.:BCN6764
CAS No.:142796-22-3
- Luteolin-3',7-di-O-glucoside
Catalog No.:BCN6380
CAS No.:257-724-7
- Isosilybin A
Catalog No.:BCN6369
CAS No.:142796-21-2
- Cistantubuloside C1
Catalog No.:BCN6362
CAS No.:620632-36-2
- Niga-ichigoside F1
Catalog No.:BCN8356
CAS No.:95262-48-9
- 1,3,6-Trihydroxy-2-methylanthraquinone 3-O-alpha-L-rhamnosyl-(1->2)-beta-D-glucoside
Catalog No.:BCN8732
CAS No.:87686-88-2
- L-Fucitol
Catalog No.:BCN8464
CAS No.:13074-06-1
- Ganoderic acid R
Catalog No.:BCN8489
CAS No.:103963-39-9
- Tessaric acid
Catalog No.:BCN8743
CAS No.:58142-10-2
- Quercetin 3-Caffeylrobinobioside
Catalog No.:BCN8744
CAS No.:957110-26-8
- Momordicine II
Catalog No.:BCN8745
CAS No.:91590-75-9
- Polygalasaponin XXXV
Catalog No.:BCN8746
CAS No.:184479-28-5
Development and validation of a rapid LC-MS/MS method for simultaneous quantification of arecoline and its two active metabolites in rat plasma and its application to a pharmacokinetic study.[Pubmed:29573735]
J Pharm Biomed Anal. 2018 May 30;154:397-403.
Arecoline is the primary active and toxic constituent of areca nut. Arecaidine and arecoline N-oxide are two major active metabolites of arecoline. In this work, an accurate and simple high performance liquid chromatography tandem mass spectrometry method for simultaneous quantification of arecoline, Arecaidine and arecoline N-oxide in rat plasma was developed and fully validated to study their pharmacokinetic behaviors in rats. After extracted from rat plasma by protein precipitation with methanol and then concentrated, the analytes were chromatographic separated on a Sepax Sapphire C18 analytical column. The mobile phase consisted of methanol and 2mM ammonium acetate buffer solution containing 0.2% (v/v) formic acid (8:92, v/v) under isocratic elution. The analytes were detected by multiple reaction monitoring (MRM) with an electrospray ionization source in the positive ion mode. The transitions of m/z 156.2-->53.2,m/z 142.2-->44.2 and m/z 172.2-->60.2 were selected for arecoline, Arecaidine and arecoline N-oxide, respectively. The method was linear over the concentration range of 0.5-100ng/mL for arecoline, 5-5000ng/mL for Arecaidine and arecoline N-oxide with no carry-over effect. The accuracies and intra- and inter-batch precisions were all within the acceptance limits. No matrix effect and potential interconversion between the analytes and other metabolites were observed in this method. The validated method was further employed to a preclinical pharmacokinetic study of arecoline, Arecaidine and arecoline N-oxide after oral treatment with 20mg/kg arecoline to rats.
M2 muscarinic receptor activation inhibits cell proliferation and migration of rat adipose-mesenchymal stem cells.[Pubmed:29227527]
J Cell Physiol. 2018 Jul;233(7):5348-5360.
Mesenchymal stem cells (MSCs), also known as stromal mesenchymal stem cells, are multipotent cells, which can be found in many tissues and organs as bone marrow, adipose tissue and other tissues. In particular MSCs derived from Adipose tissue (ADSCs) are the most frequently used in regenerative medicine because they are easy to source, rapidly expandable in culture and excellent differentiation potential into adipocytes, chondrocytes, and other cell types. Acetylcholine (ACh), the most important neurotransmitter in Central nervous system (CNS) and peripheral nervous system (PNS), plays important roles also in non-neural tissue, but its functions in MSCs are still not investigated. Although MSCs express muscarinic receptor subtypes, their role is completely unknown. In the present work muscarinic cholinergic effects were characterized in rat ADSCs. Analysis by RT-PCR demonstrates that ADSCs express M1-M4 muscarinic receptor subtypes, whereas M2 is one of the most expressed subtype. For this reason, our attention was focused on M2 subtype. By using the selective M2 against Arecaidine Propargyl Ester (APE) we performed cell proliferation and migration assays demonstrating that APE causes cell growth and migration inhibition without affecting cell survival. Our results indicate that ACh via M2 receptors, may contribute to the maintaining of the ADSCs quiescent status. These data are the first evidence that ACh, via muscarinic receptors, might contribute to control ADSCs physiology.


