Methyl LinolenateCAS# 301-00-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 301-00-8 | SDF | Download SDF |
| PubChem ID | 5319706 | Appearance | Light yellow liquid |
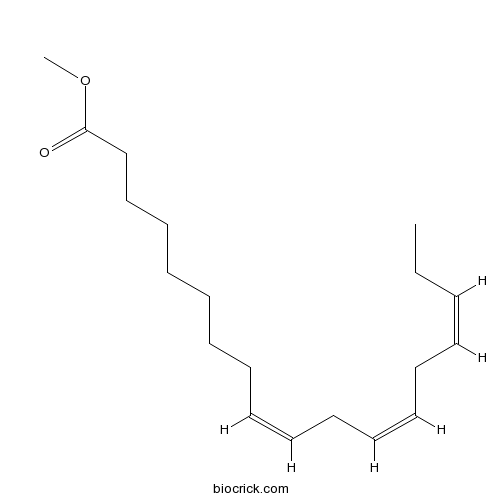
| Formula | C19H32O2 | M.Wt | 292.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (170.96 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | methyl (9Z,12Z,15Z)-octadeca-9,12,15-trienoate | ||
| SMILES | CCC=CCC=CCC=CCCCCCCCC(=O)OC | ||
| Standard InChIKey | DVWSXZIHSUZZKJ-YSTUJMKBSA-N | ||
| Standard InChI | InChI=1S/C19H32O2/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19(20)21-2/h4-5,7-8,10-11H,3,6,9,12-18H2,1-2H3/b5-4-,8-7-,11-10- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Methyl Linolenate Dilution Calculator

Methyl Linolenate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4193 mL | 17.0964 mL | 34.1927 mL | 68.3854 mL | 85.4818 mL |
| 5 mM | 0.6839 mL | 3.4193 mL | 6.8385 mL | 13.6771 mL | 17.0964 mL |
| 10 mM | 0.3419 mL | 1.7096 mL | 3.4193 mL | 6.8385 mL | 8.5482 mL |
| 50 mM | 0.0684 mL | 0.3419 mL | 0.6839 mL | 1.3677 mL | 1.7096 mL |
| 100 mM | 0.0342 mL | 0.171 mL | 0.3419 mL | 0.6839 mL | 0.8548 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Methyl linolenate is a polyunsaturated fattly acid (PUFA). It is used in studies on the mechanisms and prevention of oxidation/peroxidation of unsaturated fatty acids. [1][2] The IC50 is 60 uM. [3]
References:
[1]. Huh S et al. Melanogenesis inhibitory effect of fatty acid alkyl esters isolated from Oxalis triangularis. Biol Pharm Bull, 2010, 33(7):1242-5.
[2]. Li X et al. Benchmark Calculations for Bond Dissociation Enthalpies of Unsaturated Methyl Esters and the Bond Dissociation Enthalpies of Methyl Linolenate. J Phys Chem A, 2016, Jun 16, 120(23):4025-36
[3]. Saori Okuda et al. Impact of Lipid Physical State on the Oxidation of Methyl Linolenate in Oil-in-Water Emulsions.
- 4-CMTB
Catalog No.:BCC6250
CAS No.:300851-67-6
- GSA 10
Catalog No.:BCC6329
CAS No.:300833-95-8
- Ciluprevir (BILN-2061)
Catalog No.:BCC1482
CAS No.:300832-84-2
- RS 504393
Catalog No.:BCC1910
CAS No.:300816-15-3
- TG003
Catalog No.:BCC4416
CAS No.:300801-52-9
- BMS 309403
Catalog No.:BCC8046
CAS No.:300657-03-8
- HMBA Linker
Catalog No.:BCC2831
CAS No.:3006-96-0
- SKF 77434 hydrobromide
Catalog No.:BCC7144
CAS No.:300561-58-4
- Dehydrocorydalin
Catalog No.:BCN2474
CAS No.:30045-16-0
- Boc-Asn-ol
Catalog No.:BCC2587
CAS No.:30044-67-8
- Ro 28-1675
Catalog No.:BCC4124
CAS No.:300353-13-3
- Diphyllin O-glucoside
Catalog No.:BCN8065
CAS No.:30021-77-3
- Oleamide
Catalog No.:BCC6827
CAS No.:301-02-0
- Robinin
Catalog No.:BCN5208
CAS No.:301-19-9
- Malvidin-3-O-galactoside chloride
Catalog No.:BCN3030
CAS No.:30113-37-2
- Tianeptine sodium
Catalog No.:BCC2506
CAS No.:30123-17-2
- TCS 359
Catalog No.:BCC1183
CAS No.:301305-73-7
- CH 223191
Catalog No.:BCC3896
CAS No.:301326-22-7
- P7C3
Catalog No.:BCC6524
CAS No.:301353-96-8
- H-Val-NH2.HCl
Catalog No.:BCC3144
CAS No.:3014-80-0
- Seneganolide
Catalog No.:BCN5209
CAS No.:301530-12-1
- Compstatin control peptide
Catalog No.:BCC6067
CAS No.:301544-78-5
- Jasminin
Catalog No.:BCN7468
CAS No.:30164-93-3
- SB 431542
Catalog No.:BCC3658
CAS No.:301836-41-9
Characteristics and performance of aerobic algae-bacteria granular consortia in a photo-sequencing batch reactor.[Pubmed:29414745]
J Hazard Mater. 2018 May 5;349:135-142.
The characteristics and performance of algae-bacteria granular consortia which cultivated with aerobic granules and targeted algae (Chlorella and Scenedesmus), and the essential difference between granular consortia and aerobic granules were investigated in this experiment. The result indicated that algae-bacteria granular consortia could be successfully developed, and the algae present in the granular consortia were mainly Chlorella and Scenedesmus. Although the change of chlorophyll composition revealed the occurrence of light limitation for algal growth, the granular consortia could maintain stable granular structure, and even showed better settling property than aerobic granules. Total nitrogen and phosphate in the algal-bacterial granular system showed better removal efficiencies (50.2% and 35.7%) than those in the aerobic granular system (32.8% and 25.6%) within one cycle (6h). The biodiesel yield of aerobic granules could be significantly improved by algal coupled process, yet Methyl Linolenate and methyl palmitoleate were the dominant composition of biodiesel obtained from granular consortia and aerobic granules, respectively. Meanwhile, the difference of dominant bacterial communities in the both granules was found at the order level and family level, and alpha diversity indexes revealed the granular consortia had a higher microbial diversity.
Chemical composition, antioxidant and cytotoxic activities of extracts from the leaves of Smilax brasiliensis Sprengel (Smilacaceae).[Pubmed:28504017]
Nat Prod Res. 2018 Mar;32(5):610-615.
The antioxidant and cytotoxic activities of petroleum ether and methanol extracts, fatty acids and methyl esters from leaves of Smilax brasiliensis were evaluated, and the composition of the extracts was determined. Palmitic, linoleic and linolenic acids were major components of the extracts. For antioxidant activity, all samples exhibited IC50 values lower than BHT (2,6-di-tert-butyl-4-methylphenol). The extracts, fatty acids and methyl esters from S. brasiliensis presented no toxicity to larvae of the brine shrimp, Artemia salina. Among the purified substances, only Methyl Linolenate showed toxicity (LD50 = 21.47 mug/mL). This study showed, for the first time, the composition of petroleum ether and methanol extracts from S. brasiliensis leaves, as well as the antioxidant and cytotoxic activities of extracts, fatty acids and methyl esters.
Classification of biodiesel and fuel blends using gas chromatography - differential mobility spectrometry with cluster analysis and isolation of C18:3 me by dual ion filtering.[Pubmed:27216685]
Talanta. 2016 Aug 1;155:278-88.
Fatty acid alkyl esters (FAAEs) were determined at 10-100mg/L in biodiesel and blends with petrodiesel without sample pre-treatment using gas chromatography with a tandem differential mobility detector. Selectivity was provided through chromatographic separations and atmospheric pressure chemical ionization reactions in the detector with mobility characterization of gas ions. Limits of detection were ~0.5ng with an average of 2.98% RSD for peak area precision, Methyl Linolenate (C18:3 me) was achieved in 6-10s with a 1m long capillary column using dual ion filtering in the tandem differential mobility detector.
Benchmark Calculations for Bond Dissociation Enthalpies of Unsaturated Methyl Esters and the Bond Dissociation Enthalpies of Methyl Linolenate.[Pubmed:27191950]
J Phys Chem A. 2016 Jun 16;120(23):4025-36.
It is important to determine an appropriate computational method for obtaining accurate thermochemical properties of large biodiesel molecules such as Methyl Linolenate. In this study, we use Kohn-Sham density functional theory (DFT) and coupled cluster theory to calculate bond dissociation enthalpies (BDEs) of seven fragment molecules of Methyl Linolenate, in particular, propene, methyl formate, cis-3-hexene, 1,4-pentadiene, 1-pentene, butane, and methyl butanoate. The results are compared to BDEs obtained from experiments and to Oyeyemi et al.'s multireference averaged coupled pair functional (MRACPF2) calculations. We found that with extrapolation to the complete basis set (CBS) limit, the BDEs derived from coupled cluster calculations with single, double, and triple excitations (CCSDT) and from CCSDT with a perturbative treatment of connected quadruple excitations, CCSDT(2)Q/CBS, are closer to the available experimental values than those obtained by MRACPF2 for propene and methyl formate. The CCSDT/CBS calculations were chosen as the reference for validating the DFT methods. Among the density functionals, we found that M08-HX has the best performance with a mean unsigned deviation (MUD) from CCSDT/CBS of only 1.0 kcal/mol, whereas the much more expensive MRACPF2 has an MUD of 1.1 kcal/mol. We then used the most successfully validated density functionals to calculate the BDEs of Methyl Linolenate and compared the results with the MRACPF2 BDEs. The present study identifies several Kohn-Sham exchange-correlation functionals that should be useful for modeling ester combustion, especially the M08-HX, M06-2X, M05-2X, M08-SO, and MPWB1K global-hybrid meta functionals, the M11 and MN12-SX range-separated-hybrid meta functionals, the omegaB97 range-separated hybrid gradient approximation functional, and the SOGGA11-X global-hybrid gradient approximation functional.
Two new fatty acids esters were detected in ginseng stems by the application of azoxystrobin and the increasing of antioxidant enzyme activity and ginsenosides content.[Pubmed:27914541]
Pestic Biochem Physiol. 2016 Nov;134:63-72.
Panax ginseng C.A. Meyer is a valuable herb in China that has also gained popularity in the West because of its pharmacological properties. The constituents isolated and characterized in ginseng stems include ginsenosides, fatty acids, amino acids, volatile oils, and polysaccharides. In this study, the effects of fungicide azoxystrobin applied on antioxidant enzyme activity and ginsenosides content in ginseng stems was studied by using Panax ginseng C. A. Mey. cv. (the cultivar of Ermaya) under natural environmental conditions. The azoxystrobin formulation (25% SC) was sprayed three times on ginseng plants at different doses (150ga.i./ha and 225ga.i./ha), respectively. Two new fatty acids esters (ethyl linoleate and Methyl Linolenate) were firstly detected in ginseng stems by the application of azoxystrobin as foliar spray. The results indicated that activities of enzymatic antioxidants, the content of ginsenosides and two new fatty acids esters in ginseng stems in azoxystrobin-treated plants were increased. Azoxystrobin treatments to ginseng plants at all growth stages suggest that the azoxystrobin-induced delay of senescence is due to an enhanced antioxidant enzyme activity protecting the plants from harmful active oxygen species (AOS). The activity of superoxide dismutase (SOD) in azoxystrobin-treated plants was about 1-3 times higher than that in untreated plants. And the effects was more significant (P=0.05) when azoxystrobin was applied at dose of 225ga.i./ha. This work suggests that azoxystrobin plays an important role in delaying of senescence by changing physiological and biochemical indicators and increasing ginsenosides content in ginseng stems.
Evaluation of phytochemicals from medicinal plants of Myrtaceae family on virulence factor production by Pseudomonas aeruginosa.[Pubmed:28294414]
APMIS. 2017 May;125(5):482-490.
Virulence factors regulated by quorum sensing (QS) play a critical role in the pathogenesis of an opportunistic human pathogen, Pseudomonas aeruginosa in causing infections to the host. Hence, in the present work, the anti-virulence potential of the medicinal plant extracts and their derived phytochemicals from Myrtaceae family was evaluated against P. aeruginosa. In the preliminary screening of the tested medicinal plant extracts, Syzygium jambos and Syzygium antisepticum demonstrated a maximum inhibition in QS-dependent violacein pigment production by Chromobacterium violaceum DMST 21761. These extracts demonstrated an inhibitory activity over a virulence factor, pyoverdin, production by P. aeruginosa ATCC 27853. Gas chromatography-mass spectrometric (GC-MS) analysis revealed the presence of 23 and 12 phytochemicals from the extracts of S. jambos and S. antisepticum respectively. Three top-ranking phytochemicals, including phytol, ethyl linoleate and Methyl Linolenate, selected on the basis of docking score in molecular docking studies lowered virulence factors such as pyoverdin production, protease and haemolytic activities of P. aeruginosa to a significant level. In addition, the phytochemicals reduced rhamnolipid production by the organism. The work demonstrated an importance of plant-derived compounds as anti-virulence drugs to conquer P. aeruginosa virulence towards the host.


