LopinavirHIV protease inhibitor,highly potent CAS# 192725-17-0 |
- Deuterated Atazanivir-D3-2
Catalog No.:BCC2116
CAS No.:1092540-51-6
- Deuterated Atazanivir-D3-1
Catalog No.:BCC2115
CAS No.:1092540-56-1
- Saquinavir
Catalog No.:BCC1921
CAS No.:127779-20-8
- Saquinavir mesylate
Catalog No.:BCC1922
CAS No.:149845-06-7
- Darunavir
Catalog No.:BCC3623
CAS No.:206361-99-1
- Oleanolic acid
Catalog No.:BCN5616
CAS No.:508-02-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 192725-17-0 | SDF | Download SDF |
| PubChem ID | 92727 | Appearance | Powder |
| Formula | C37H48N4O5 | M.Wt | 628.81 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ABT-378 | ||
| Solubility | DMSO : 100 mg/mL (159.03 mM; Need ultrasonic) | ||
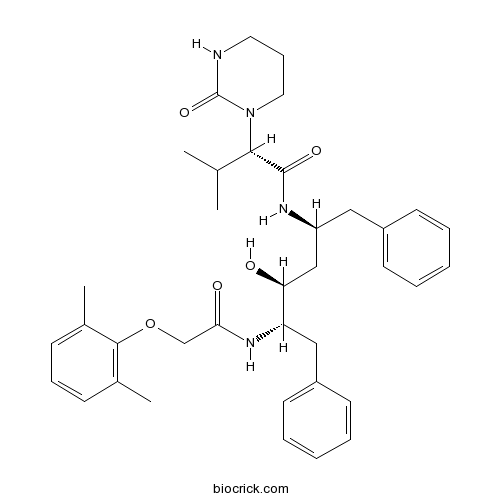
| Chemical Name | (2S)-N-[(2S,4S,5S)-5-[[2-(2,6-dimethylphenoxy)acetyl]amino]-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide | ||
| SMILES | CC1=C(C(=CC=C1)C)OCC(=O)NC(CC2=CC=CC=C2)C(CC(CC3=CC=CC=C3)NC(=O)C(C(C)C)N4CCCNC4=O)O | ||
| Standard InChIKey | KJHKTHWMRKYKJE-SUGCFTRWSA-N | ||
| Standard InChI | InChI=1S/C37H48N4O5/c1-25(2)34(41-20-12-19-38-37(41)45)36(44)39-30(21-28-15-7-5-8-16-28)23-32(42)31(22-29-17-9-6-10-18-29)40-33(43)24-46-35-26(3)13-11-14-27(35)4/h5-11,13-18,25,30-32,34,42H,12,19-24H2,1-4H3,(H,38,45)(H,39,44)(H,40,43)/t30-,31-,32-,34-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Lopinavir is a potent HIV protease inhibitor with Ki of 1.3 pM. | |||||
| Targets | HIV protease | |||||
| IC50 | 1.3 pM (Ki) | |||||

Lopinavir Dilution Calculator

Lopinavir Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5903 mL | 7.9515 mL | 15.9031 mL | 31.8061 mL | 39.7576 mL |
| 5 mM | 0.3181 mL | 1.5903 mL | 3.1806 mL | 6.3612 mL | 7.9515 mL |
| 10 mM | 0.159 mL | 0.7952 mL | 1.5903 mL | 3.1806 mL | 3.9758 mL |
| 50 mM | 0.0318 mL | 0.159 mL | 0.3181 mL | 0.6361 mL | 0.7952 mL |
| 100 mM | 0.0159 mL | 0.0795 mL | 0.159 mL | 0.3181 mL | 0.3976 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Lopinavir (also known as ABT-378) is a highly potent inhibitor of human immunodeficiency virus (HIV) protease that potently inhibits wild-type and mutant HIV protease with inhibition constant Ki values ranging from 1.3 to 3.6 pM. Lopinavir, a ritonavir analog designed to have a diminished interaction with Val82 in HIV protease, maintains a high potency inhibiting Val82 mutant HIV selected by ritonavir with 50% effective concentration EC50 value lower than 0.06 μM. Although the antiviral activity of ritonavir is considerably attenuated by human serum, lopinavir is less affected by human serum proteins and is 10-fold greater in potency than ritonavir in the presence of human serum.
Reference
Sham HL, Kempf DJ, Molla A, Marsh KC, Kumar GN, Chen CM, Kati W, Stewart K, Lal R, Hsu A, Betebenner D, Korneyeva M, Vasavanonda S, McDonald E, Saldivar A, Wideburg N, Chen X, Niu P, Park C, Jayanti V, Grabowski B, Granneman GR, Sun E, Japour AJ, Leonard JM, Plattner JJ, Norbeck DW. ABT-378, a highly potent inhibitor of the human immunodeficiency virus protease. Antimicrob Agents Chemother. 1998 Dec;42(12):3218-24.
- C527
Catalog No.:BCC3972
CAS No.:192718-06-2
- PD 161570
Catalog No.:BCC7765
CAS No.:192705-80-9
- H-DL-HoSer-OH
Catalog No.:BCC3242
CAS No.:1927-25-9
- 9-Benzoylcarbazole
Catalog No.:BCC8799
CAS No.:19264-68-7
- PPADS tetrasodium salt
Catalog No.:BCC6725
CAS No.:192575-19-2
- 11α-Hydroxycanrenone
Catalog No.:BCC8433
CAS No.:192569-17-8
- 9alpha,11,12-Trihydroxydrim-7-en-6-one
Catalog No.:BCN7388
CAS No.:192566-65-7
- Ergosta-4,6,8(14),22-tetraen-3-one
Catalog No.:BCN1183
CAS No.:19254-69-4
- Lomeguatrib
Catalog No.:BCC1133
CAS No.:192441-08-0
- Neuropeptide SF (human)
Catalog No.:BCC5829
CAS No.:192387-39-6
- Neuropeptide AF (human)
Catalog No.:BCC5854
CAS No.:192387-38-5
- Prazosin HCl
Catalog No.:BCC2505
CAS No.:19237-84-4
- 3-Isomangostin
Catalog No.:BCN1214
CAS No.:19275-46-8
- Cudraflavone B
Catalog No.:BCN8067
CAS No.:19275-49-1
- Cyclomulberrin
Catalog No.:BCN3374
CAS No.:19275-51-5
- ES 936
Catalog No.:BCC6102
CAS No.:192820-78-3
- 2-Amino-2'-nitrodiphenyl sulfide
Catalog No.:BCC8522
CAS No.:19284-81-2
- LY310762
Catalog No.:BCC5052
CAS No.:192927-92-7
- Ximelagatran
Catalog No.:BCC6382
CAS No.:192939-46-1
- ZM 323881 HCl
Catalog No.:BCC5098
CAS No.:193000-39-4
- ZM323881
Catalog No.:BCC2073
CAS No.:193001-14-8
- CTS-1027
Catalog No.:BCC1502
CAS No.:193022-04-7
- Cardamonin
Catalog No.:BCN1184
CAS No.:19309-14-9
- Otamixaban
Catalog No.:BCC1827
CAS No.:193153-04-7
Comparative pharmacokinetic evaluation of lopinavir and lopinavir-loaded solid lipid nanoparticles in hepatic impaired rat model.[Pubmed:28317117]
J Pharm Pharmacol. 2017 Jul;69(7):823-833.
OBJECTIVE: Drug-induced hepatotoxicity is a major cause of concern in patients receiving HIV/TB co-treatment. Lopinavir (LPV), an anti-HIV drug, shows poor plasma exposure due to hepatic first-pass metabolism. In this study, we investigated the effect of hepatotoxicity on pharmacokinetics of free LPV and LPV-loaded solid lipid nanoparticles (LPV SLNs) in male Wistar rats. METHODS: Hepatic impairment model in rats was developed by injecting CCl4 (i.p., 2 ml/kg). Comparative pharmacokinetic (n = 5) and tissue distribution studies (n = 3) were conducted following oral administration (20 mg/kg) of free LPV and LPV SLNs in normal and hepatic impaired rats. Isolated perfused liver (IPL) model (n = 3) and cycloheximide intervened lymphatic uptake studies (n = 3) were conducted to appreciate disposition pattern of LPV. KEY FINDINGS: In contrary to free LPV, pharmacokinetic results demonstrated no significant (P > 0.05) difference in drug plasma profile of LPV SLNs in normal and impaired rats. IPL model demonstrated trivial role of liver in disposition of LPV SLNs. Tissue distribution studies of SLNs showed higher (P < 0.05) LPV accumulation in lymphoidal organs. Pretreatment of cycloheximide significantly (P < 0.05) reduced AUC and Cmax of LPV SLNs. CONCLUSION: From the results, we conclude that unlike conventional formulations of LPV, disposition characteristics of LPV SLNs were similar both in normal and hepatic impaired rats.
Simultaneous LC-MS-MS Determination of Lopinavir and Rifabutin in Human Plasma.[Pubmed:28334925]
J Chromatogr Sci. 2017 Jul 1;55(6):617-624.
Tuberculosis (TB) with human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome represents the most common infectious diseases worldwide. Anti-TB drugs are used concurrently with antiretroviral drug for treatment of TB-HIV co-morbidities. Due to lower risk of interaction with protease inhibitors, rifabutin is preferred over rifampicin in treatment of HIV and TB co-morbidity. A simple and specific liquid chromatography tandem mass spectrometry method was developed for quantification of rifabutin (RBT) and Lopinavir (LPV) simultaneously in human plasma. Following extraction using 60% n-hexane in ethyl acetate, the processed samples were chromatographed on a Discovery HS C18 column (5 mum, 50 x 4.6 mm, id) using mobile phase [85% acetonitrile in ammonium acetate buffer (10 mM, pH 4.5)] at a flow rate of 0.7 mL/min. Mass spectrometric detection was performed in positive electrospray ionization mode using multiple reaction monitoring (RBT, m/z 847.7 --> 815.4; LPV, m/z 629.6 --> 447.4). Raloxifene and phenacetin were used as internal standards for RBT and LPV, respectively. Linearity was established in the range of 1-1,000 ng/mL and 0.5-10 microg/mL (R2 >/= 0.99) for RBT and LPV, respectively. The recovery of LPV and RBT were always >90 and >50%, respectively. The precisions and accuracies were well within the acceptable limits of variation.
Impact of lopinavir-ritonavir exposure in HIV-1 infected children and adolescents in Madrid, Spain during 2000-2014.[Pubmed:28350802]
PLoS One. 2017 Mar 28;12(3):e0173168.
BACKGROUND: The most-used protease-inhibitor in children is Lopinavir-ritonavir (LPV/r), which provides durable suppression of viral load and increases CD4+T-counts. This study describes the virological outcome of the HIV-1-infected paediatric population exposed to LPV/r during 15 years in Spain. METHODOLOGY: Patients from the Madrid Cohort of HIV-1-infected-children and adolescents exposed to LPV/r as different line therapy during 2000-2014 were selected. The baseline epidemiological-clinical features, viral suppression, changes in CD4+T-CD8+T cell counts and drug susceptibility were recorded before and during LPV/r exposure. Drug resistance mutations (DRM) were identified in viruses from samples collected until 2011. We predicted drug susceptibility to 19 antiretrovirals among those carrying DRM using the Stanford's HIVdb Algorithm. RESULTS: A total of 199 (37.3%) of the 534 patients from the cohort were exposed to LPV/r during 2000-2014 in first (group 1), second (group 2) or more line-therapies (group 3). Patients were mainly Spaniards (81.9%), perinatally infected (96.5%) with subtype-B (65.3%) and HIV-diagnosed before year 2000 (67.8%). The mean age at first LPV/r exposure was 9.7 years. After protease-inhibitor exposure, viral suppression was higher in groups 1 and 2 than in group 3. Viral suppression occurred in 87.5%, 68.6% and 64.8% patients from groups 1, 2 and 3, respectively. Among the 64 patients with available resistance data during LPV/r treatment, 27(42.3%) carried DRM to protease-inhibitor, 28 (58.3%) to reverse-transcriptase-inhibitors and 21 (43.7%) to non-reverse-transcriptase-inhibitors. Darunavir/ritonavir, atazanavir-ritonavir and tipranavir/ritonavir presented the highest susceptibility and nelfinavir the lowest. CONCLUSIONS: A better lymphocyte recovering occurred when protease-inhibitor was taken as part of a first-line regimen and a higher number of patients reached viral suppression. The least compromised antiretrovirals for rescue antiretroviral regimens, according to DRM in the LPV/r-exposed-paediatric cohort, were mainly the new protease inhibitors.


